Batch-fabricated full glassy carbon fibers for real-time tonic and phasic dopamine detection
- PMID: 40092374
- PMCID: PMC11906454
- DOI: 10.3389/fbioe.2025.1543882
Batch-fabricated full glassy carbon fibers for real-time tonic and phasic dopamine detection
Abstract
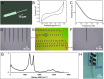
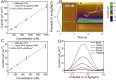
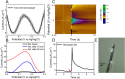
Dopamine (DA) is a critical neurotransmitter that is key in regulating motor functions, motivation, and reward-related behavior. Measuring both tonic (baseline, steady-state) and phasic (rapid, burst-like) DA release is essential for elucidating the mechanisms underlying neurological disorders, such as schizophrenia and Parkinson's disease, which are associated with dysregulated tonic and phasic DA signaling. Carbon fiber microelectrodes (CFEs) are considered the gold standard for measuring rapid neurotransmitter changes due to their small size (5-10 µm), biocompatibility, flexibility, and excellent electrochemical properties. However, achieving consistent results and large-scale production of CFE arrays through manual fabrication poses significant challenges. We previously developed flexible glassy carbon (GC) microelectrode arrays (MEAs) and GC fiber-like MEAs (GCF MEAs) for neurotransmitter detection and electrophysiology recording. We also demonstrated the feasibility of fabricating GC MEA with both GC electrodes and interconnects made from a single homogeneous material, eliminating the need for metal interconnections and addressing related concerns about electrical and mechanical stability under prolonged electrochemical cycling. Building on our prior experience, we now present a double-etching microfabrication technique for the batch production of 10 μm × 10 µm full GC fibers (fGCFs) and fGCF arrays, composed entirely of homogeneous GC material. This process uses a 2 µm-thick low-stress silicon nitride as the bottom insulator layer for the fGCFs. The effectiveness of the fabrication process was validated through scanning electron microscophy (SEM) and energy dispersive X-ray spectroscopy (EDS) elemental analyses, which confirmed the uniformity of the Si₃N₄ insulation layer and ensured the overall integrity of the fGCFs. Using finite element analysis, we optimized the fGCF form factor to achieve self-penetration up to 3 mm into the mouse striatum without additional support. The electrochemical characterization of fGCFs demonstrated high electrical conductivity and a wide electrochemical window. The ability of fGCFs to detect phasic and tonic DA release was confirmed using fast scan cyclic voltammetry (FSCV) and square wave voltammetry (SWV), respectively, both in vitro and in vivo. With their high sensitivity for phasic and tonic DA detection, combined with a scalable fabrication process and self-supporting insertion capability, fGCFs are promising sensors that offer enhanced practicality for comprehensive DA monitoring.
Keywords: dopamine; fast scan cyclic voltammetry (FSCV); glassy carbon fibers; microelectrodes; square wave voltammetry (SWV).
Copyright © 2025 Siwakoti, Pwint, Broussard, Rivera, Cui and Castagnola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Arana L. R., de Mas N., Schmidt R., Franz A. J., Schmidt M. A., Jensen K. F. (2007). Isotropic etching of silicon in fluorine gas for MEMS micromachining. J. Micromechanics Microengineering 17 (2), 384–392. 10.1088/0960-1317/17/2/026 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

