This is a preprint.
An intranasal adjuvanted, recombinant influenza A/H5 vaccine candidate induces broad priming against diverse influenza A/H5N1 virus clades in a phase I randomized trial in healthy adults
- PMID: 40092447
- PMCID: PMC11908355
- DOI: 10.21203/rs.3.rs-6059149/v1
An intranasal adjuvanted, recombinant influenza A/H5 vaccine candidate induces broad priming against diverse influenza A/H5N1 virus clades in a phase I randomized trial in healthy adults
Abstract
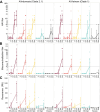
We conducted a randomized, controlled phase I trial (NCT05397119) of a novel adjuvanted recombinant influenza A/H5 (A/Indonesia/05/2005, clade 2.1) hemagglutinin vaccine, administered intranasally in two doses 28 days apart at three antigen levels. Control groups received unadjuvanted recombinant H5 or formulation buffer placebo. Six months later, participants received a heterologous unadjuvanted inactivated influenza A/H5N1 (A/Vietnam/1203/2004, clade 1) vaccine intramuscularly. All vaccines were safe and well tolerated. After the primary intranasal series, serum hemagglutination inhibition and microneutralization responses were minimal. Increases in mucosal and serum IgG/IgA, serum surface plasmon resonance antibody binding, memory B cell and CD4 T cell activity, and antibody-dependent cell-mediated cytotoxicity were observed only in recipients primed intranasally with adjuvanted H5 vaccine. Following the inactivated H5N1 boost, robust responses across all immune assays, as well as microneutralization responses against diverse H5N1 clades (including currently circulating clade 2.3.4.4b), occurred in adjuvanted vaccine recipients, demonstrating successful priming and broad responses.
Conflict of interest statement
MED: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. FRT: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. MP: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. HG: None. SK: None. TH: Employee of BlueWillow Biologics, Inc at time of study design. AF: Employee of BlueWillow Biologics, Inc at time of study design. YL: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. SMT: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. MFM: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. PJB: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. JJO: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. SD: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. JPB: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. LC: None to disclose. KMN: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. CC: Is an employee of BlueWillow Biologics, Inc. KLK: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. MBS: Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study. JRO: Personal: Advisory Boards for GSK: (RSV Cost Effectiveness Ad Board, Vaccine Virtual Days 2023), Pfizer: Pfizer’s Advisory Committee of Influenza Experts (ACTIvE), and Moderna: New Vaccines Advisory Board. Consultant ENA Respiratory. Institution has received research funds from BlueWillow Biologics, Inc. to support assays performed for this study.
Figures





References
-
- White E.B., et al. Influenza Vaccine Effectiveness Against Illness and Asymptomatic Infection in 2022–2023: A Prospective Cohort Study. Clin Infect Dis (2024). - PubMed
-
- Paules C.I., Marston H.D., Eisinger R.W., Baltimore D. & Fauci A.S. The Pathway to a Universal Influenza Vaccine. Immunity 47, 599–603 (2017). - PubMed
-
- Neuzil K.M., et al. Data and product needs for influenza immunization programs in low- and middle-income countries: Rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine 35, 5734–5737 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

