Fostering kappa (κ)-carrageenan hydrogels with the power of a natural crosslinker: a comparison between tender coconut water and potassium chloride (KCl) for therapeutic applications
- PMID: 40092448
- PMCID: PMC11908996
- DOI: 10.1007/s13205-025-04254-0
Fostering kappa (κ)-carrageenan hydrogels with the power of a natural crosslinker: a comparison between tender coconut water and potassium chloride (KCl) for therapeutic applications
Abstract
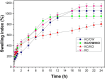
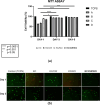
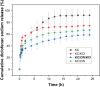
This study investigated the potential of tender coconut water as a natural alternative to potassium chloride (KCl) to crosslink κ-carrageenan hydrogels. κ-Carrageenan hydrogels crosslinked with tender coconut water, KCl, and their combination were formulated with diclofenac sodium as model drug, and their morphology, chemical bonding, compressive strength, water uptake capacity, degradation resistance, and cytotoxicity were assessed. The results showed that crosslinking κ-carrageenan hydrogels with both tender coconut water and KCl increased their compressive strength by up to 450%, provided excellent water retention capacity, and resulted in only 5% degradation after 20 days. Scanning electron microscopy revealed that crosslinking the hydrogel with both tender coconut water and KCl compacted its morphological structure, which remained biocompatible when tested with 3T3 cells. Infrared analysis confirmed that incorporated diclofenac sodium remained inert during preparation of the hydrogel matrices. Furthermore, the in vitro release behavior and antimicrobial properties of the hydrogels were assessed. The drug release profile from hydrogels crosslinked with both tender coconut water and KCl was sustained over 24 h. Such hydrogels also showed a unique antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli)-with the activity against E. coli being more pronounced. In conclusion, these results confirm that crosslinking with tender coconut water and KCl is a superior alternative to just with KCl for κ-carrageenan hydrogels.
Supplementary information: The online version contains supplementary material available at 10.1007/s13205-025-04254-0.
Keywords: Antibacterial activity; Biocompatibility; Diclofenac sodium; Drug delivery; Polymer matrix; Polysaccharides; Tissue engineering.
© The Author(s) 2025.
Conflict of interest statement
Conflict of interestThe authors have no conflicts of interest to declare. The authors declare that they have no conflict of interest in the publication.
Figures




References
-
- Adeyeye CM, Li PK (1990) Diclofenac Sodium. Analytical Profiles of Drug Substances and Excipients, Elsevier 19:123–144. 10.1016/S0099-5428(08)60366-4
-
- Algharib SA, Dawood A, Zhou K et al (2020) Designing, structural determination and biological effects of rifaximin loaded chitosan- carboxymethyl chitosan nanogel. Carbohydr Polym 248:116782. 10.1016/J.CARBPOL.2020.116782 - PubMed
-
- Bakarich SE, Balding P, Gorkin R et al (2014) Printed ionic-covalent entanglement hydrogels from carrageenan and an epoxy amine. RSC Adv 4:38088–38092. 10.1039/C4RA07109C
-
- Balasubramaniam MP, Murugan P, Chenthamara D et al (2020) Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater Today Commun 25:101510. 10.1016/J.MTCOMM.2020.101510
-
- Bibire T, Yilmaz O, Ghiciuc CM et al (2022) Biopolymers for surgical applications. Coatings 12:211. 10.3390/COATINGS12020211
LinkOut - more resources
Full Text Sources

