The Novel Long-Acting Peptide S6-FA Attenuates Liver Fibrosis In Vitro and In Vivo
- PMID: 40092780
- PMCID: PMC11904669
- DOI: 10.1021/acsomega.4c10956
The Novel Long-Acting Peptide S6-FA Attenuates Liver Fibrosis In Vitro and In Vivo
Abstract
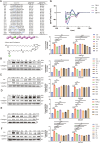
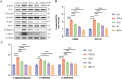
Liver fibrosis can progress to cirrhosis and hepatocellular carcinoma. Currently, there is no effective drug for liver fibrosis. The peptide 6 (T6) is an endogenous peptide derived from human intrauterine adhesion tissues and has antifibrotic potential. Here, to improve the long-term efficacy and activity of T6, we conducted the rational modified of T6 through studying structure-activity, and synthesized a series of analogues. Among them, S6 and S6-FA exhibited optimal antihepatic fibrosis activity, and S6-FA had a stronger long-acting effect than T6 and S6. The two analogues inhibited the expression of α-SMA and Collagen 1 in TGF-β-induced LX2 cells model and CCl4-induced mouse model of liver fibrosis. Besides, we discovered that S6 and S6-FA remarkably reduced the AST and ALT serum levels. Mechanistic studies have demonstrated that analogues inhibited liver fibrosis through inhibiting Erk, Smad and P65 pathways. This study provided that the novel peptide S6 and S6-FA is potential candidate compounds for treating liver fibrosis.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Xu H.; Zhao Q.; Song N.; Yan Z.; Lin R.; Wu S.; Jiang L.; Hong S.; Xie J.; Zhou H.; Wang R.; Jiang X. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020, 11 (1), 580710.1038/s41467-020-19668-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
