Glassy Fluorene and Sulfone Polyesters with High Refractive Index and Thermal Stability Whose Monomers Induce Estrogenic Bioactivity
- PMID: 40092783
- PMCID: PMC11904644
- DOI: 10.1021/acsomega.4c10782
Glassy Fluorene and Sulfone Polyesters with High Refractive Index and Thermal Stability Whose Monomers Induce Estrogenic Bioactivity
Abstract
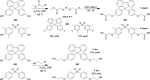
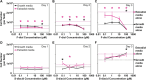
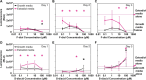
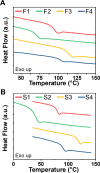
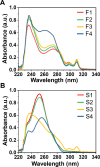
Two new diacid monomers containing either fluorene or sulfone moieties polymerize via step-growth polymerization in the presence of their diol counterparts or alkyl diols (ethylene glycol and 1,6-hexanediol), forming eight new cardo structure polyesters. The polymers exhibit high optical transparency in the thin-film form with refractive indices ranging from 1.56 to 1.69, tunable glass transition temperatures from ca. 40 to 116 °C with no melting temperature, and resistance to thermal degradation in a nitrogenous atmosphere, reaching 350-398 °C before observing 10% weight loss. Their molecular weights, M w , range from ca. 17 to 77 kDa, with an average polydispersity, Đ, of 1.5, and average purified yields of 82%. The polymers absorb light primarily in the UV region from ca. 228 to 320 nm with no absorption from 320 to 800 nm. A critical finding is that some of the fluorene and sulfone monomers induce negative bioactivity and even cell death in T47D-KBLuc cells, a breast cancer cell line, at high concentrations. This report details the project inspiration, monomer synthesis, polymerization steps, structural confirmation, material characterization, and the impact of the new monomer's estrogenic and antiestrogenic bioactivity.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Multicomponent Polymerizations Provide Sustainable Sulfur (Selenium)-Containing Polyesters.Acc Chem Res. 2025 Apr 15;58(8):1345-1353. doi: 10.1021/acs.accounts.5c00152. Epub 2025 Mar 31. Acc Chem Res. 2025. PMID: 40163816
-
Synthesis of Biobased Poly(butylene Furandicarboxylate) Containing Polysulfone with Excellent Thermal Resistance Properties.Biomacromolecules. 2024 Mar 11;25(3):1825-1837. doi: 10.1021/acs.biomac.3c01272. Epub 2024 Feb 9. Biomacromolecules. 2024. PMID: 38336482
-
Bio-Based Degradable Poly(ether-ester)s from Melt-Polymerization of Aromatic Ester and Ether Diols.Int J Mol Sci. 2022 Aug 11;23(16):8967. doi: 10.3390/ijms23168967. Int J Mol Sci. 2022. PMID: 36012244 Free PMC article.
-
Recent Advances in the Development of 1,4-Cyclohexanedimethanol (CHDM) and Cyclic-Monomer-Based Advanced Amorphous and Semi-Crystalline Polyesters for Smart Film Applications.Materials (Basel). 2024 Sep 17;17(18):4568. doi: 10.3390/ma17184568. Materials (Basel). 2024. PMID: 39336309 Free PMC article. Review.
-
Terephthalic Acid Copolyesters Containing Tetramethylcyclobutanediol for High-Performance Plastics.ChemistryOpen. 2021 Aug;10(8):830-841. doi: 10.1002/open.202100171. ChemistryOpen. 2021. PMID: 34402603 Free PMC article. Review.
References
-
- Shi Y.; Wang W.; Lin W.; Olson D. J.; Bechtel J. H. Double-End Crosslinked Electro-Optic Polymer Modulators with High Optical Power Handling Capability. Appl. Phys. Lett. 1997, 70 (11), 1342–1344. 10.1063/1.118574. - DOI
-
- Hreha R. D.; Haldi A.; Domercq B.; Barlow S.; Kippelen B.; Marder S. R. Synthesis of Acrylate and Norbornene Polymers with Pendant 2,7-Bis(Diarylamino)Fluorene Hole-Transport Groups. Tetrahedron. 2004, 60 (34), 7169–7176. 10.1016/j.tet.2004.06.069. - DOI
-
- Vallejos S.; Estévez P.; García F. C.; Serna F.; De La Peña J. L.; García J. M. Putting to Work Organic Sensing Molecules in Aqueous Media: Fluorene Derivative-Containing Polymers as Sensory Materials for The Colorimetric Sensing of Cyanide In Water. Chem. Commun. 2010, 46 (42), 7951.10.1039/c0cc02143a. - DOI - PubMed
LinkOut - more resources
Full Text Sources
