Reduced plasma levels of GM-CSF is a common feature of Schistosoma mansoni-infected school-aged children
- PMID: 40092989
- PMCID: PMC11906694
- DOI: 10.3389/fimmu.2025.1474575
Reduced plasma levels of GM-CSF is a common feature of Schistosoma mansoni-infected school-aged children
Abstract
Background: Currently available schistosomiasis diagnostic and monitoring tools are limited, and the development of novel technologies is necessary to enhance disease diagnostic and surveillance by supporting elimination efforts. Novel disease-specific biomarkers can facilitate the development of these technologies. Through the comparison of parasite burden and host factors, we assessed whether host plasma cytokines could be used as robust biomarkers for intestinal schistosomiasis and associated pathology in school-aged children (SAC) living in endemic areas.
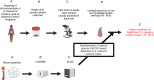
Methods: Levels of host plasma cytokines were measured in SAC from a low-to-moderate burden region five months deworming with praziquantel, using Luminex assay for exploration analysis and ELISA for validation.
Results: The concentration of GM-CSF, IL-2, and VEGF in plasma was significantly lower in schistosome-infected compared to non-infected children, as determined by Luminex assay. Further evaluation by ELISA revealed a negative correlation between GM-CSF plasma levels, but not those of IL-2 or VEGF, and S. mansoni egg burdens in infected individuals. Common coinfections in the study area such as geohelminths, hepatitis or malaria failed to alter plasma GM-CSF levels arguing in favor of a potential specific effect of S. mansoni infection on this cytokine. Receiver operating characteristic analysis confirmed GM-CSF as an acceptable predictive marker of S. mansoni infection, with an area under the curve (AUC) of 75%. Finally, the adjunct use of plasmatic GM-CSF thresholds for screening S. mansoni at-risk children and identify S. mansoni-infected ones increased the sensitivity of a single Kato-Katz test by averagely 15%.
Conclusions: Our findings highlight the potential of using plasma GM-CSF levels to biomark S. mansoni infection and improve the sensitivity of single Kato-Katz based diagnostic for low- to-moderate burden infections.
Keywords: GM-CSF; adjunct diagnostic; biomarker; cytokine; schistosomiasis.
Copyright © 2025 Kamdem, Kamguia, Oumarou, Bitye, Lennard, Brombacher, Spangenberg, Demarta-Gatsi and Nono.
Conflict of interest statement
Authors TS and CD-G were employed by the company Ares Trading S.A., a subsidiary of Merck KGaA, Darmstadt, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Clements AC, Brooker S, Nyandindi U, Fenwick A, Blair L. Bayesian spatial analysis of a national urinary schistosomiasis questionnaire to assist geographic targeting of schistosomiasis control in Tanzania, East Africa. Int J Parasitol. (2008) 38:401–15. doi: 10.1016/j.ijpara.2007.08.001 - DOI - PMC - PubMed
-
- Wiegand RE, Fleming FM, de Vlas SJ, Odiere MR, Kinung'hi S, King CH, et al. . Defining elimination as a public health problem for schistosomiasis control programmes: beyond prevalence of heavy-intensity infections. Lancet Glob Health. (2022) 10:e1355–e9. doi: 10.1016/S2214-109X(22)00287-X - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

