Beyond CCR7: dendritic cell migration in type 2 inflammation
- PMID: 40093008
- PMCID: PMC11906670
- DOI: 10.3389/fimmu.2025.1558228
Beyond CCR7: dendritic cell migration in type 2 inflammation
Abstract
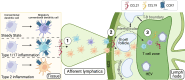
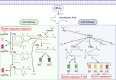
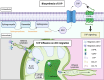
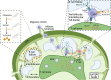
Conventional dendritic cells (cDCs) are crucial antigen-presenting cells that initiate and regulate T cell responses, thereby shaping immunity against pathogens, innocuous antigens, tumors, and self-antigens. The migration of cDCs from peripheral tissues to draining lymph nodes (dLNs) is essential for their function in immune surveillance. This migration allows cDCs to convey the conditions of peripheral tissues to antigen-specific T cells in the dLNs, facilitating effective immune responses. Migration is primarily mediated by chemokine receptor CCR7, which is upregulated in response to homeostatic and inflammatory cues, guiding cDCs to dLNs. However, during type 2 immune responses, such as those triggered by parasites or allergens, a paradox arises-cDCs exhibit robust migration to dLNs despite low CCR7 expression. This review discusses how type 2 inflammation relies on additional signaling pathways, including those induced by membrane-derived bioactive lipid mediators like eicosanoids, sphingolipids, and oxysterols, which cooperate with CCR7 to enhance cDC migration and T helper 2 (Th2) differentiation. We explore the potential regulatory mechanisms of cDC migration in type 2 immunity, offering insights into the differential control of cDC trafficking in diverse immune contexts and its impact on immune responses.
Keywords: 7α,25-Dihydroxycholesterol (7α,25-OHC); CCR7; EBI2 (GPR183); cysteinyl leukotrienes (cysLTs); dendritic cell migration; prostaglandins (PGs); sphingosine-1-phosphate (S1P); type 2 immune responses.
Copyright © 2025 Meloun and León.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

