This is a preprint.
SARS-CoV-2 Fusion Peptide-Directed Antibodies Elicited by Natural Infection Mediate Broad Sarbecovirus Neutralization
- PMID: 40093263
- PMCID: PMC11908342
- DOI: 10.1101/2025.03.01.25323010
SARS-CoV-2 Fusion Peptide-Directed Antibodies Elicited by Natural Infection Mediate Broad Sarbecovirus Neutralization
Abstract
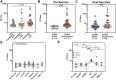
Studies have demonstrated that repeated mRNA vaccination enhances the breadth of neutralization against diverse SARS-CoV-2 variants. However, the development of antibodies capable of neutralizing across the Coronavirinae subfamily is poorly understood. In this study, we analyze serum samples to determine their neutralization breadth and potency and identify their antigenic targets. Using a cohort of older individuals and healthcare workers, we track correlates of broad neutralizing responses, including fusion peptide (FP) antibody elicitation. We find that although broadly neutralizing responses are often a result of RBD-specific antibodies, a rare subset of donors produce FP-specific broadly neutralizing responses. Interestingly, FP-specific antibodies are not observed in COVID-naive individuals irrespective of vaccination regimen, but rather, they occur following natural infection or vaccine breakthrough. This study highlights the epitope targets underpinning broadly neutralizing antibody responses to coronaviruses and suggests that existing vaccines are insufficient to promote the elicitation of FP-directed broadly neutralizing coronavirus antibodies.
Keywords: COVID-19; SARS-COV-2; broad neutralization; fusion peptide; neutralizing antibodies; stem helix.
Conflict of interest statement
DECLARATION OF INTERESTS A.B.B. is a founder of Cure Systems LLC. S. G. and D. H. C. are recipients of investigator-initiated grants to their universities from Pfizer to study pneumococcal vaccines, Sanofi Pasteur and Seqirus to study influenza vaccines, Moderna to study respiratory infection, and GSK to study herpes zoster. S. G. is the recipient of an investigator-initiated grant to the university from Genentech on influenza antivirals; reports consulting for GSK, Janssen/Johnson&Johnson, Merck, Novavax, Moderna, Seqirus, Sanofi, and Vaxart; he has received honoraria for speaking for AstraZeneca, Pfizer, Seqirus, Sanofi; reports personal fees from Pfizer; and reports data and safety monitoring board fees from Longevoron and SciClone.
Figures




References
-
- COVID - Coronavirus Statistics - Worldometer. https://www.worldometers.info/coronavirus/#google_vignette.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
