Development, biological evaluation, and molecular modelling of novel isocytosine and guanidine derivatives as BACE1 inhibitors using a fragment growing strategy
- PMID: 40093519
- PMCID: PMC11904611
- DOI: 10.1039/d4md00698d
Development, biological evaluation, and molecular modelling of novel isocytosine and guanidine derivatives as BACE1 inhibitors using a fragment growing strategy
Abstract
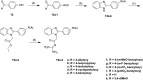
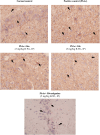
Alzheimer's disease (AD) is a neurodegenerative condition characterized by significant synaptic loss and neuronal death in brain regions critical for cognitive functions. The disease is characterized by the formation of amyloid plaques, which are extracellular constructs consisting mainly of aggregated Aβ42. The latter is a peptide formed by the proteolytic cleavage of β-amyloid precursor protein (APP) by two enzymes, β- and γ-secretase. Therefore, inhibition of the aspartic protease β-secretase (BACE1) is considered a promising therapeutic approach for the treatment and prevention of Alzheimer's disease. Unfortunately, a limited number of β-secretase inhibitors have reached human trials and eventually failed due to inconclusive therapeutic and/or safety profiles. In this study, we developed drug-like molecules with a β-secretase inhibitory activity using a fragment growing strategy on isocytosine and acyl guanidine warheads. Our approach is based on optimizing the hydrophobic part of the molecules to obtain a conformationally restrained scaffold complementary to the hydrophobic pockets within the enzyme active site. We developed 32 compounds with promising in vitro inhibitory activity against BACE1 down to sub-micromolar IC50. Docking simulation studies were performed to understand the mode of binding of the prepared compounds. We demonstrated that compounds with superior activities, such as 16b and 16g, are able to provide the best balance between the steric shape and position of the polar substituent for achieving preferential anchoring into the S1, S3, S1', and S2' sub-pockets. Further, in vivo characterization of selected drug-like candidates of the benzimidazole series AMK-IV, namely 16a and 16k, demonstrated their ability to reduce oxidation stress and their safety within brain and liver tissues.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interest.
Figures











References
-
- Skaria A. P. Am. J. Manag. Care. 2022;28(10 Suppl):S188–S196. - PubMed
LinkOut - more resources
Full Text Sources

