In vivo neuroprotection in ischemic stroke by activated protein C requires β-arrestin 2
- PMID: 40093572
- PMCID: PMC11911019
- DOI: 10.1016/j.bvth.2024.100037
In vivo neuroprotection in ischemic stroke by activated protein C requires β-arrestin 2
Abstract
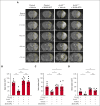
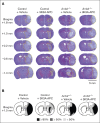
The protease activated protein C (APC) and its variants provide neuroprotection for murine ischemic stroke and mortality reduction for murine sepsis. For these actions, APC's in vivo mechanism of action, similar to in vitro studies using cultured cells, involves protease activated receptor 1 (PAR1)-mediated biased signaling. APC/PAR1 signaling in vitro requires β-arrestin 2, an intracellular scaffold protein, and β-arrestin 2-initiated signaling can alter diverse intracellular signaling pathways. This study used a proximal transient middle cerebral artery occlusion model to study the neuroprotective actions of the signaling-selective APC variant, 3K3A-APC, in β-arrestin 2-deficient (Arrb2 -/-) mice. Based on quantitation of brain injuries, 3K3A-APC significantly limited brain injury in control mice to relatively small, localized areas, whereas 3K3A-APC's protection was lost in Arrb2 -/- mice. Thus, the major in vitro mechanism of action that requires β-arrestin 2 for APC/PAR1 biased signaling is central to the in vivo mechanism of action for APC's neuroprotection.
Conflict of interest statement
Conflict-of-interest disclosure: J.H.G. is a coinventor for Scripps-owned patents related to some studies in this report. B.V.Z. is the scientific cofounder of ZZ Biotech LLC. B.V.Z. and J.H.G. are on the scientific advisory board of ZZ Biotech LLC. The remaining authors declare no competing financial interests.
Figures

References
-
- O'Donnell JS, Fleming H, Noone D, Preston RJS. Unraveling coagulation factor-mediated cellular signaling. J Thromb Haemost. 2023;21(12):3342–3353. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
