Rapid and cumulative adult plasticity in the mouse visual cortex
- PMID: 40093861
- PMCID: PMC11906431
- DOI: 10.3389/fncir.2025.1537305
Rapid and cumulative adult plasticity in the mouse visual cortex
Abstract
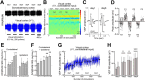
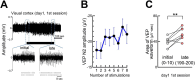
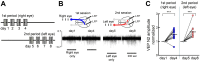
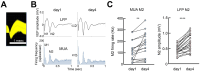
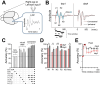
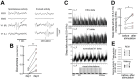
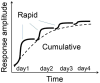
Experience-dependent neural plasticity enables the brain to adapt to diverse and dynamic environments by reshaping circuits. In the adult visual system, this plasticity can be elicited by repeated sensory stimuli; however, its temporal dynamics and underlying mechanisms remain unclear. Here, we investigated the regulation of visual response potentiation induced by repeated light flashes in the primary visual cortex of awake adult mice. Our findings revealed two distinct temporal phases of potentiation: a rapid phase occurring within seconds and a cumulative phase developing over hours to days. Notably, the identification of this rapid phase phenomenon adds to and refines the prevailing view that visual plasticity in the adult cortex is predominantly slow. Additionally, exposure to visual stimuli enhanced spontaneous slow-wave activity in the visual cortex during non-REM sleep. This plasticity was significantly impaired in Grin2a (NR2A) knockout mice, a model of schizophrenia, which mirrors visual plasticity deficits observed in human patients. The dual temporal characteristics of flash-evoked visual plasticity likely reflect multifaceted aspects of adult brain functionality, encompassing processes related to memory, learning, and neurological disorders. This model of visual plasticity in defined neural circuits provides a simplified yet robust and extensible framework for exploring the neural mechanisms underlying adaptive and maladaptive behavioral changes.
Keywords: NMDA receptors; NREM sleep; experience-dependent adult plasticity; flash-evoked potentials; mouse visual cortex; stimulus-selective response plasticity (SRP).
Copyright © 2025 Miyamoto, Mazaki, Makino, Fang, Hamada, Handa and Hensch.
Conflict of interest statement
QF, TH, and YH were employed by the Daikin Industries, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



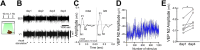
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

