Sex- and age-specific sensitivities of the endocannabinoid system in Alzheimer's disease revealed by PET imaging with [18F]FMPEP- d 2 and [18F]MAGL-2102
- PMID: 40093888
- PMCID: PMC11905134
- DOI: 10.7150/thno.106592
Sex- and age-specific sensitivities of the endocannabinoid system in Alzheimer's disease revealed by PET imaging with [18F]FMPEP- d 2 and [18F]MAGL-2102
Abstract
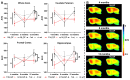
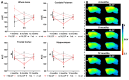
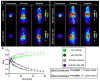
The endocannabinoid system is a critical brain signaling pathway that is dysregulated in various brain disorders, including Alzheimer's disease (AD). Cannabinoid-targeted therapies and imaging approaches have gained increasing interest; however, the biological impact of the endocannabinoid system in disease needs further validation. We aimed to study changes in cannabinoid receptor 1 (CB1) and monoacylglycerol lipase (MAGL), components of endocannabinoid signaling and degradation, in a mouse model of AD by PET imaging. Methods: [18F]FMPEP-d 2 and [18F]MAGL-2102 were produced on a commercial radiosynthesis module. PET-CT images with both tracers were acquired in a knock-in mouse model of AD bearing mutated human amyloid precursor protein (AppNL-G-F ) at 3 ages, and compared to wild-type mice. Excised brains were used for in vitro autoradiography with [18F]FMPEP-d 2 and [18F]MAGL-2102, immunofluorescence, and western blotting. Male wild-type and 5xFAD mice were chronically treated with MAGL inhibitor JZL184 and imaged with [18F]MAGL-2102 two days after ending treatment. Results: PET imaging showed sex-, age- and genotype-dependent changes in CB1 and MAGL availability. At 4-months (early-stage β-amyloid pathology), female AppNL-G-F mice had lower CB1 availability, and MAGL availability was increased in male AppNL-G-F , compared to wild-types. At 8-months, no genotype differences in CB1 were observed, yet MAGL availability was reduced in AppNL-G-F frontal cortex, and male AppNL-G-F mice exhibited higher MAGL than transgenic females brain-wide. At 12-months (late-stage β-amyloid pathology), significantly lower uptake of [18F]FMPEP-d 2 was observed in AppNL-G-F compared to wild-type, with no changes in [18F]MAGL-2102 binding. AppNL-G-F plaque staging was confirmed by Thioflavin-S staining. Imaging findings were supplemented by autoradiography, immunofluorescence, and western blots. [18F]MAGL-2102 availability was responsive to target engagement of the MAGL inhibitor JZL184 in wild-type and 5xFAD mice. Conclusions: The present study showed dynamic age-, sex- and pathology-related changes in CB1 and MAGL availability from early-stage β-amyloid pathology, suggesting that the endocannabinoid system is a useful target for diagnostics and treatment of AD. Finally, these results highlight that endocannabinoid sex differences should be considered in diagnostics and drug development.
Keywords: Alzheimer's disease; PET; cannabinoid receptor 1; endocannabinoid system; monoacylglycerol lipase.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

