Comparison of 5-aminolevulinic acid and MMP-14 targeted peptide probes in preclinical models of GBM
- PMID: 40093889
- PMCID: PMC11905129
- DOI: 10.7150/thno.107210
Comparison of 5-aminolevulinic acid and MMP-14 targeted peptide probes in preclinical models of GBM
Abstract
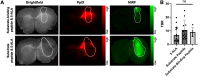
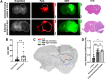
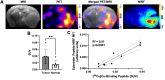
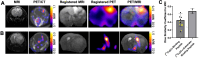
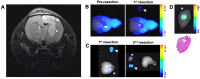
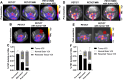
Rationale: Developing novel pre-operative and intraoperative imaging approaches for glioblastoma multiforme (GBM) could aid therapeutic intervention while sparing healthy normal brain, which remains a significant clinical challenge. 5-aminolevulinic acid (5-ALA) is the only intraoperative imaging agent approved to aid the resection of GBM. Matrix metalloproteinase 14 (MMP14), which is overexpressed in GBM, is an attractive target for preoperative and intraoperative imaging of GBM. Prior studies have shown the feasibility of near-infrared fluorescence (NIRF) imaging and positron emission tomography (PET) imaging of GBM xenografts in mice using MMP-14 targeted peptide probes. The present studies assessed the tumor-specific localization and contrast of these MMP-14 targeted peptides relative to 5-ALA in GBM models. Methods: Fluorescence and PET imaging was performed after i.v. injection of 5-ALA and the MMP-14 targeted peptide probes (non-labeled or radiolabeled with 64Cu) in mice bearing human GBM orthotopic xenografts (U87, D54). Imaging signals were correlated to MMP-14 expression determined by immunofluorescence. Tumor-to-normal brain ratio (TBR) and Dice similarity coefficient (DSC) relative to tumor defined by ex vivo pathology or in vivo magnetic resonance imaging were determined for each imaging agent. Results: NIRF signals from the MMP-14 targeted peptide probes showed comparable TBR (p < 0.05) but significantly higher DSC (p < 0.05) relative to 5-ALA. NIRF signals from the peptide probes significantly correlated with MMP-14 expression (p < 0.05). MMP-14 binding peptide labeled with 64Cu showed moderate DSC (0.45) while PET signals significantly correlated (p < 0.05) with NIRF signals from a co-injected MMP-14 substrate peptide. NIRF and PET signals localized in residual tumor regions in the resection cavity during in situ resection. Conclusions: MMP-14 targeted peptides showed favorable TBR and higher tumor localization than 5-ALA in GBM orthotopic models. Further development of MMP-14 targeted peptide probes could lead to improved pre-operative and intraoperative management of GBM.
Keywords: 5-ALA; GBM; MMP-14; NIRF; PET.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–71. - PubMed
-
- Daniel Orringer, Darryl Lau, Sameer Khatri, Grettel J. Zamora-Berridi, Kathy Zhang, Chris Wu, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–9. - PubMed
-
- Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124:977–88. - PubMed
-
- Farrell C, Shi W, Bodman A, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines update on the role of emerging developments in the management of newly diagnosed glioblastoma. J Neurooncol. 2020;150:269–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

