Engineered Probiotic-Based Biomaterials for Inflammatory Bowel Disease Treatment
- PMID: 40093907
- PMCID: PMC11905135
- DOI: 10.7150/thno.103983
Engineered Probiotic-Based Biomaterials for Inflammatory Bowel Disease Treatment
Abstract
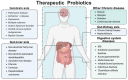
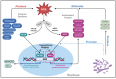
Inflammatory bowel disease (IBD) is a chronic condition affecting the intestines, marked by immune-mediated inflammation. This disease is known for its recurrent nature and the challenges it presents in treatment. Recently, probiotic have gained attention as a promising alternative to traditional small molecular drugs and monoclonal antibody chemotherapies for IBD. Probiotic, recognized as a "living" therapeutic agent, offers targeted treatment with minimal side effects and the flexibility for biological modifications, making them highly effective for IBD management. This comprehensive review presents the latest advancements in engineering probiotic-based materials, ranging from basic treatment mechanisms to the modification techniques used in IBD management. It delves deep into how probiotic produces therapeutic effects in the intestinal environment and discusses various strategies to enhance probiotic's efficacy, including genetic modifications and formulation improvements. Additionally, the review addresses the challenges, practical application conditions, and future research directions of probiotic-based therapies in IBD treatment, providing insights into their feasibility and potential clinical implications.
Keywords: engineered probiotic-based materials; inflammatory bowel disease; living materials; probiotics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures









References
-
- Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN. et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: An international organization for study of inflammatory bowel diseases consensus. Lancet Gastroenterol Hepatol. 2022;7:666–78. - PubMed
-
- Ledder O, Turner D. Antibiotics in IBD: Still a role in the biological era? Inflamm Bowel Dis. 2018;24:1676–88. - PubMed
-
- Núñez F P, Quera R, Bay C, Castro F, Mezzano G. Drug-induced liver injury used in the treatment of inflammatory bowel disease. J Crohns Colitis. 2022;16:1168–76. - PubMed
-
- Kaakoush NO. Fecal transplants as a microbiome-based therapeutic. Curr Opin Microbiol. 2020;56:16–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

