Genomic Screening at a Single Health System
- PMID: 40094662
- PMCID: PMC11915069
- DOI: 10.1001/jamanetworkopen.2025.0917
Genomic Screening at a Single Health System
Abstract
Importance: Completion of the Human Genome Project prompted predictions that genomics would transform medicine, including through genomic screening that identifies potentially medically actionable findings that could prevent disease, detect it earlier, or treat it better. However, genomic screening remains anchored in research and largely unavailable as part of routine care.
Objective: To summarize 11 years of experience with genomic screening and explore the landscape of genomic screening efforts.
Design, setting, and participants: This cohort study was based in Geisinger's MyCode Community Health Initiative, a genomic screening program in a rural Pennsylvania health care system in which patient-participants exomes are analyzed.
Main outcomes and measures: Genomic screen-positive rates were evaluated and stratified by condition type (cancer, cardiovascular, other) and US Centers for Disease Control and Prevention (CDC) Tier 1 designation. The proportion of participants previously unaware of their genomic result was assessed. Other large-scale population-based genomic screening efforts with genomic results disclosure were compiled from public resources.
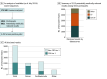
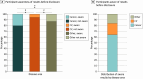
Results: A total of 354 957 patients participated in Geisinger's genomic screening program (median [IQR] age, 54 [36-69] years; 194 037 [59.7%] assigned female sex at birth). As of June 2024, 175 500 participants had exome sequencing available for analysis, and 5934 participants (3.4%) had a pathogenic variant in 81 genes known to increase risk for disease. Between 2013 and July 2024, 5119 results were disclosed to 5052 eligible participants, with 2267 (44.2%) associated with risk for cardiovascular disease, 2031 (39.7%) with risk for cancer, and 821 (16.0%) with risk for other conditions. Most results (3040 [59.4%]) were in genes outside of those with a CDC Tier 1 designation. Nearly 90% of participants (4425 [87.6%]) were unaware of their genomic risk prior to disclosure. In a survey of large-scale biobanks with genomic and electronic health record (EHR) data, only 25.0% (6 of 24) disclosed potentially actionable genomic results.
Conclusions and relevance: In this large, genomics-informed cohort study from a single health system, 1 in 30 participants had a potentially actionable genomic finding. However, nearly 90% were unaware of their risk prior to screening, demonstrating the utility of genomic screening in identifying at-risk individuals. Most large-scale biobanks with genomic and EHR data did not return genomic results with potential medical relevance, missing opportunities to significantly improve genomic risk ascertainment for these individuals and to perform longitudinal studies of clinical and implementation outcomes in diverse settings.
Conflict of interest statement
Figures

Comment in
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

