Donepezil for Fatigue and Psychological Symptoms in Post-COVID-19 Condition: A Randomized Clinical Trial
- PMID: 40094666
- PMCID: PMC11915061
- DOI: 10.1001/jamanetworkopen.2025.0728
Donepezil for Fatigue and Psychological Symptoms in Post-COVID-19 Condition: A Randomized Clinical Trial
Abstract
Importance: Fatigue is the most commonly reported symptom of post-COVID-19 condition (also known as long COVID) and impairs various functions. One of the underlying mechanisms may be intracerebral inflammation due to decreases in acetylcholine levels.
Objective: To examine the effects of donepezil hydrochloride, an acetylcholinesterase inhibitor, on post-COVID-19 fatigue and psychological symptoms.
Design, setting, and participants: A multicenter, double-blind randomized clinical trial was performed in Japan. Between December 14, 2022, and March 31, 2024, adult patients within 52 weeks of the onset of COVID-19 and with a global binary fatigue score of 4 or greater on the Chalder Fatigue Scale were randomized into a donepezil or a placebo group.
Exposure: The intervention was conducted during a 3-week period, with donepezil hydrochloride being administered at a dosage of 3 mg/d for the first week and then 5 mg/d for 2 weeks.
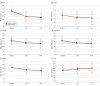
Main outcomes and measures: The primary outcome was a change in the Chalder Fatigue Scale score and the absolute score 3 weeks after the initiation of treatment. Other outcomes at 3 and 8 weeks, such as psychological symptoms and quality of life, were evaluated as secondary outcomes.
Results: A total of 120 eligible patients were enrolled and 10 withdrew or were lost to follow-up; therefore, 110 patients (55 in each group) were included in the efficacy analysis (64 [58%] female; mean [SD] age, 43 [12] years). No significant differences were observed in baseline characteristics between the 2 groups. The baseline-adjusted estimating treatment effect of donepezil, measured as the mean difference on Chalder Fatigue Scale scores at 3 weeks, was 0.34 (95% CI, -2.23 to 2.91), showing no significant effect of the intervention (P = .79). Scores for the Hospital Anxiety and Depression Scale, Impact of Event Scale-Revised, EuroQol 5-Dimension 5-Level Version, Patient Health Questionnaire, and Daily Health Status at 3 and 8 weeks were similar. No serious adverse events occurred in either group.
Conclusions and relevance: In this randomized clinical trial of donepezil to treat post-COVID-19 condition, the efficacy for fatigue and psychological symptoms was not confirmed in a general population. The development of effective therapeutics for post-COVID-19 symptoms is needed, and more clinical trials should be conducted in the future.
Trial registration: Japan Registry of Clinical Trials Identifier: jRCT 2031220510.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

