Automated Imaging Differentiation for Parkinsonism
- PMID: 40094699
- PMCID: PMC11915115
- DOI: 10.1001/jamaneurol.2025.0112
Automated Imaging Differentiation for Parkinsonism
Abstract
Importance: Magnetic resonance imaging (MRI) paired with appropriate disease-specific machine learning holds promise for the clinical differentiation of Parkinson disease (PD), multiple system atrophy (MSA) parkinsonian variant, and progressive supranuclear palsy (PSP). A prospective study is needed to test whether the approach meets primary end points to be considered in a diagnostic workup.
Objective: To assess the discriminative performance of Automated Imaging Differentiation for Parkinsonism (AIDP) using 3-T diffusion MRI and support vector machine (SVM) learning.
Design, setting, and participants: This was a prospective, multicenter cohort study conducted from July 2021 to January 2024 across 21 Parkinson Study Group sites (US/Canada). Included were patients with PD, MSA, and PSP with established criteria and unanimous agreement in the clinical diagnosis among 3 independent, blinded neurologists who specialize in movement disorders. Patients were assigned to a training set or an independent testing set.
Exposure: MRI.
Main outcomes and measures: Area under the receiver operating characteristic curve (AUROC) in the testing set for primary model end points of PD vs atypical parkinsonism, MSA vs PSP, PD vs MSA, and PD vs PSP. AIDP was also paired with antemortem MRI to test against postmortem neuropathology in a subset of autopsy cases.
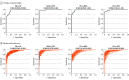
Results: A total of 316 patients were screened and 249 patients (mean [SD] age, 67.8 [7.7] years; 155 male [62.2%]) met inclusion criteria. Of these patients, 99 had PD, 53 had MSA, and 97 had PSP. A retrospective cohort of 396 patients (mean [SD] age, 65.8 [8.9] years; 234 male [59.1%]) was also included. Of these patients, 211 had PD, 98 had MSA, and 87 had PSP. Patients were assigned to the training set (78%; 104 prospective, 396 retrospective) or independent testing set, which included 145 (22%; 60 PD, 27 MSA, 58 PSP) prospective patients (mean age, 67.4 [SD 7.7] years; 95 male [65.5%]). The model was robust in differentiating PD vs atypical parkinsonism (AUROC, 0.96; 95% CI, 0.93-0.99; positive predictive value [PPV], 0.91; negative predictive value [NPV], 0.83), MSA vs PSP (AUROC, 0.98; 95% CI, 0.96-1.00; PPV, 0.98; NPV, 0.81), PD vs MSA (AUROC, 0.98; 95% CI, 0.96-1.00; PPV, 0.97; NPV, 0.97), and PD vs PSP (AUROC, 0.98; 95% CI, 0.96-1.00; PPV, 0.92; NPV, 0.98). AIDP predictions were confirmed neuropathologically in 46 of 49 brains (93.9%).
Conclusions and relevance: This prospective multicenter cohort study of AIDP met its primary end points. Results suggest using AIDP in the diagnostic workup for common parkinsonian syndromes.
Conflict of interest statement
Figures


References
-
- Lehericy S, Vaillancourt DE, Seppi K, et al. ; International Parkinson and Movement Disorder Society (IPMDS)-Neuroimaging Study Group . The role of high-field magnetic resonance imaging in parkinsonian disorders: pushing the boundaries forward. Mov Disord. 2017;32(4):510-525. doi:10.1002/mds.26968 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

