Interneuron migration impairment and brain region-specific DNA damage response following irradiation during early neurogenesis in mice
- PMID: 40095026
- PMCID: PMC11914712
- DOI: 10.1007/s00018-025-05643-7
Interneuron migration impairment and brain region-specific DNA damage response following irradiation during early neurogenesis in mice
Erratum in
-
Correction: Interneuron migration impairment and brain region-specific DNA damage response following irradiation during early neurogenesis in mice.Cell Mol Life Sci. 2025 Jun 19;82(1):244. doi: 10.1007/s00018-025-05749-y. Cell Mol Life Sci. 2025. PMID: 40537529 Free PMC article. No abstract available.
Abstract
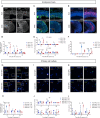
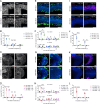
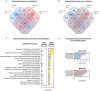
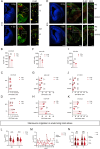
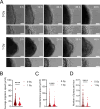
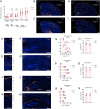
Embryonic DNA damage resulting from DNA repair deficiencies or exposure to ionizing radiation during early neurogenesis can lead to neurodevelopmental disorders, including microcephaly. This has been linked to an excessive DNA damage response in dorsal neural progenitor cells (NPCs), resulting in p53-dependent apoptosis and premature neuronal differentiation which culminates in depletion of the NPC pool. However, the effect of DNA damage on ventral forebrain NPCs, the origin of interneurons, remains unclear. In this study, we investigated the sequelae of irradiation of mouse fetuses at an early timepoint of forebrain neurogenesis. We focused on the neocortex (NCX) and medial ganglionic eminence (MGE), key regions for developing dorsal and ventral NPCs, respectively. Although both regions showed a typical p53-mediated DNA damage response consisting of cell cycle arrest, DNA repair and apoptosis, NCX cells displayed prolonged cell cycle arrest, while MGE cells exhibited more sustained apoptosis. Moreover, irradiation reduced the migration speed of interneurons in acute living brain slices and MGE explants, the latter indicating a cell-intrinsic component in the defect. RNA sequencing and protein analyses revealed disruptions in actin and microtubule cytoskeletal-related cellular machinery, particularly in MGE cells. Despite massive acute apoptosis and an obvious interneuron migration defect, prenatally irradiated animals did not show increased sensitivity to pentylenetetrazole-induced seizures, nor was there a reduction in cortical interneurons in young adult mice. This suggests a high plasticity of the developing brain to acute insults during early neurogenesis. Overall, our findings indicate that embryonic DNA damage induces region-specific responses, potentially linked to neurodevelopmental disorders.
Keywords: DNA damage; Interneuron migration; Microcephaly; Neurodevelopment; Seizures.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All procedures involving animals were in compliance with the Belgian laboratory animal legislation and the European Communities Council Directive (2010/63/EU), and approved by the local ethical committees at SCK CEN (Medanex Clinic, EC MxCl 2020 − 164), Hasselt University and Vrije Universiteit Brussel. Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- Dunn K, Yoshimaru H, Otake M, Annegers JF, Schull WJ (1990) Prenatal exposure to ionizing radiation and subsequent development of seizures. Am J Epidemiol 131:114–123 - PubMed
-
- Lin DD, Roper SN (2017) Chap. 58 - Utero irradiation as a model of cortical dysplasia. In: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshé SL (Eds.) Models of seizures and epilepsy (2nd edn). Academic Press, pp 877–885
-
- Miller MW (1996) Effect of early exposure to ethanol on the protein and DNA contents of specific brain regions in the rat. Brain Res 734:286–294 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

