Access Options for Transcatheter Aortic Valve Replacement
- PMID: 40095640
- PMCID: PMC11900295
- DOI: 10.3390/jcm14051651
Access Options for Transcatheter Aortic Valve Replacement
Abstract
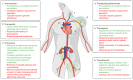
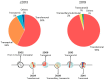
Transcatheter aortic valve replacement (TAVR) was introduced in 2002 and has become integral in the management of aortic stenosis. As an alternative to surgical aortic valve replacement, it relies heavily on safe access to the aortic annulus for implantation of a valve prosthesis. Throughout its development and in current practice, the transfemoral (TF) arterial route for retrograde valve delivery has been the primary approach. However, this route is not appropriate for all patients, which has led to the development of multiple alternate access options. This review discusses the development of access for TAVR, followed by a thorough discussion of TF access. The commercially available products, preprocedural planning, closure techniques, and procedural complications are all discussed. We also describe the various alternate access routes with particular emphasis on the most recently developed route, transcaval access (TCv), with focus on procedural indications, technical considerations, and comparative outcomes. As TAVR technology, indications, and availability all expand, the knowledge and implementation of safe access are of utmost importance.
Keywords: arterial access; transaortic; transapical; transaxillary; transcarotid; transcatheter aortic valve replacement; transcaval; transfemoral.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Cribier A., Eltchaninoff H., Bash A., Borenstein N., Tron C., Bauer F., Derumeaux G., Anselme F., Laborde F., Leon M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.CIR.0000047200.36165.B8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

