Cystathionine β-Synthase Deficiency in the E-HOD Registry-Part II: Dietary and Pharmacological Treatment
- PMID: 40095936
- PMCID: PMC11729643
- DOI: 10.1002/jimd.12844
Cystathionine β-Synthase Deficiency in the E-HOD Registry-Part II: Dietary and Pharmacological Treatment
Abstract
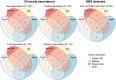
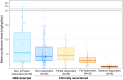
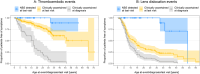
Cystathionine β-synthase (CBS) deficiency (classical homocystinuria) has a wide range of severity. Mildly affected patients typically present as adults with thromboembolism and respond to treatment with pyridoxine. Severely affected patients usually present during childhood with learning difficulties, ectopia lentis and skeletal abnormalities; they are pyridoxine non-responders (NR) or partial responders (PR) and require treatment with a low-methionine diet and/or betaine. The European network and registry for Homocystinurias and methylation Defects (E-HOD) has published management guidelines for CBS deficiency and recommended keeping plasma total homocysteine (tHcy) concentrations below 100 μmol/L. We have now analysed data from 311 patients in the registry to see how closely treatment follows the guidelines. Pyridoxine-responsive patients generally achieved tHcy concentrations below 50 μmol/L, but many NRs and PRs had a mean tHcy considerably above 100 μmol/L. Most NRs were managed with betaine and a special diet. This usually involved severe protein restriction and a methionine-free amino acid mixture, but some patients had a natural protein intake substantially above the WHO safe minimum. Work is needed on the methionine content of dietary protein as estimates vary widely. Contrary to the guidelines, most NRs were on pyridoxine, sometimes at dangerously high doses. tHcy concentrations were similar in groups prescribed high or low betaine doses and natural protein intakes. High tHcy levels were probably often due to poor compliance. Comparing time-to-event graphs for NR patients detected by newborn screening and those ascertained clinically showed that treatment could prevent thromboembolism (risk ratio 0.073) and lens dislocation (risk ratio 0.069).
Keywords: betaine; homocystinuria; methionine; newborn screening; protein restriction; pyridoxine.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Andrew A. M. Morris received an honorarium for a lecture from Travere and subsequently provided consultancy to Travere, for which honoraria were paid to E‐HOD funds. Stefan Kölker declares that a Dietmar Hopp Foundation grant was awarded to his institution. Carlo Dionisi‐Vici received consulting fees from Mamoxi and participated in Advisory Board of Nutricia and Vitaflo. Matthias R. Baumgartner declares that a research grant from Nutricia Milupa GmbH was awarded to his institution. Martina Huemer declares consultancy honoraria or travel support from Nutricia Metabolics, Sanofi, Chiesi and Immedica Pharma, all unrelated to this work. Her institution has received unrestricted research grants from Nutricia Metabolics, Sanofi and Travere. Viktor Kožich provided consultancy to GAIN Therapeutic and Travere, honoraria were paid to E‐HOD funds. The other authors declare no conflicts of interest. E‐HOD has been receiving support for its activities from Aeglea, Gain Therapeutic, Nutricia Metabolics, Recordati, SOBI, Travere and Vitaflo. The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Figures



References
-
- Kožich V., Sokolová J., Morris A. A. M., et al., “Cystathionine β‐Synthase Deficiency in the E‐HOD Registry‐Part I: Pyridoxine Responsiveness as a Determinant of Biochemical and Clinical Phenotype at Diagnosis,” Journal of Inherited Metabolic Disease 44 (2021): 677–692, 10.1002/jimd.12338. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

