Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis
- PMID: 40096083
- PMCID: PMC11949351
- DOI: 10.1371/journal.ppat.1012363
Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis
Abstract
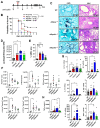
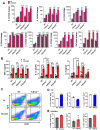
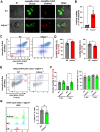
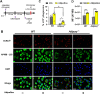
Although innate immunity is critical for antifungal host defense against the human opportunistic fungal pathogen Aspergillus fumigatus, potentially damaging inflammation must be controlled. Adiponectin (APN) is an adipokine produced mainly in adipose tissue that exerts anti-inflammatory effects in adipose-distal tissues such as the lung. We observed increased mortality and increased fungal burden and inflammation in neutropenic mice with invasive aspergillosis (IA) that lack APN or the APN receptors AdipoR1 or AdipoR2. Alveolar macrophages (AMs), early immune sentinels that detect and respond to lung infection, express both receptors, and APN-deficient AMs exhibited an inflammatory phenotype that was associated with decreased fungal killing. Pharmacological stimulation of AMs with AdipoR agonist AdipoRon rescued deficient killing in APN-/- AMs and was dependent on the presence of either receptor. Finally, APN-enhanced fungal killing was associated with increased activation of the non-canonical LC3 pathway of autophagy. Thus, our study identifies a novel role for APN in LC3-mediated killing of A.fumigatus.
Copyright: © 2025 Goli et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Update of
-
Adiponectin pathway activation dampens inflammation and enhances alveolar macrophage fungal killing via LC3-associated phagocytosis.bioRxiv [Preprint]. 2024 Aug 24:2024.06.24.600373. doi: 10.1101/2024.06.24.600373. bioRxiv. 2024. Update in: PLoS Pathog. 2025 Mar 17;21(3):e1012363. doi: 10.1371/journal.ppat.1012363. PMID: 38979340 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

