Large-scale profiling of antibody reactivity to glycolipids in patients with Guillain-Barré syndrome
- PMID: 40096525
- PMCID: PMC12588681
- DOI: 10.1093/brain/awaf102
Large-scale profiling of antibody reactivity to glycolipids in patients with Guillain-Barré syndrome
Abstract
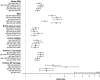
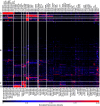
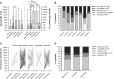
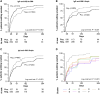
Guillain-Barré syndrome is an acute polyradiculoneuropathy in which preceding infections often elicit the production of antibodies that target peripheral nerve antigens, principally gangliosides. Anti-ganglioside antibodies are thought to play a key role in the clinical diversity of the disease and can be helpful in clinical practice. Extensive research into clinical associations of individual anti-ganglioside antibody specificities has been performed. Recent research has highlighted glycolipid complexes, glycolipid combinations that may alter antibody binding, as targets. In this study, we investigated antibody reactivity patterns to glycolipids and glycolipid complexes using combinatorial array, in relation to clinical features in Guillain-Barré syndrome. In total, 1413 patients from the observational International Guillain-Barré syndrome Outcome Study (0-91 years, 60.3% male) and 1061 controls (healthy, family, infectious, vaccination, other neurological disease) were included. Acute-phase sera from patients were screened for IgM, IgG, and IgA reactivity against 15 glycolipids and one phospholipid and their heteromeric complexes, similarly to archived control sera. Antibody specificities and reactivity patterns were analysed in relation to clinical features. Of all patients, 1309 (92.6%) were positive for at least one anti-glycolipid (complex) antibody. Anti-GM1 and anti-GQ1b (complex) antibodies best distinguished motor Guillain-Barré syndrome and Miller Fisher syndrome from controls, with antibodies to glycolipid complexes outperforming antibodies to single glycolipids. Three models consisting of anti-glycolipid (complex) antibodies distinguished patients with Guillain-Barré syndrome, the motor variant, and Miller Fisher syndrome from controls with high sensitivity and specificity, performing better than antibodies to single glycolipids used in clinical practice. Seven patient clusters with particular antibody reactivity patterns were identified. These clusters were distinguished by geographical region, clinical variants, preceding Campylobacter jejuni infection, electrophysiological subtypes, the Medical Research Council sum score at study entry, and the ability to walk 10 m unaided at 26 weeks. Two patient clusters with distinct anti-GM1 (complex) reactivity (broad versus restricted) differed in frequency of the axonal subtype. In cumulative incidence analyses, 15 anti-glycolipid (complex) antibodies were associated with the time required to regain the ability to walk 10 m unaided. After adjustment for known prognostic factors, IgG anti-GQ1b:GM4, GQ1b:PS and GQ1b:Sulfatide remained associated with faster recovery. Addition of anti-glycolipid antibodies to clinical prognostic models slightly improved their discriminative capacity, though insufficiently to improve the models. Measurement of anti-glycolipid antibodies by combinatorial array increases the diagnostic yield compared to assaying single glycolipids, identifies clinically relevant antibody reactivity patterns to glycolipids and glycolipid complexes, and may be useful in outcome prediction in Guillain-Barré syndrome.
Keywords: autoantibody; peripheral neuropathy.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
V.G. is currently an employee of Biohaven Pharmaceuticals. RDMH received honoraria from Takeda, CSL Behring, ArgenX, and Dianthus Therapeutics. J.K.L.H. has served on advisory boards and received support to attend conferences from CSL Behring and Takeda outside the submitted work. Su.Ku. received honoraria from CSL Behring, Japan Blood Product Organization, Takeda Pharmaceuticals, and KMBiologics; served on the data and safety monitoring board for ArgenX. Sa.Ku. received honoraria from CSL Behring, ArgenX, and Takeda Pharmaceuticals outside the submitted work. M.K. received speaker honoraria from CSL Behring, Japan Blood Product Organization, and Takeda Pharmaceuticals. L.Q. received speaker or expert testimony honoraria from CSL Behring, Novartis, Sanofi-Genzyme, Merck, Annexon, Alnylam, Janssen, ArgenX, UCB, Dianthus Therapeutics, LFB, Avilar Therapeutics, Nuvig Therapeutics, Takeda, and Roche; was supported by Instituto de Salud Carlos III—Ministry of Economy and Innovation (Spain), CIBERER, Fundació La Marató, GBS-CIDP Foundation International, UCB, ArgenX, and Grifols; serves at Clinical Trial Steering Committees for Sanofi Genzyme, Takeda, and ArgenX and was Principal Investigator for UCB's CIDP01 trial. P.R. served on advisory boards for UCB, ArgenX, Biogen, Alexion, and Roche outside the submitted work. KAS was supported by Grifols (Grifols Investigator-Sponsored Research, 31 August 2015-30 August 2017). R.H. was supported by GBS-CIDP Foundation International and the T2B collaboration project funded by PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public-private partnerships and co-financing by health foundations that are part of the SGF.Health∼Holland. The remaining authors report no competing interests.
Figures

References
-
- Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397:1214–1228. - PubMed
-
- Kusunoki S, Willison HJ, Jacobs BC. Antiglycolipid antibodies in Guillain-Barré and Fisher syndromes: Discovery, current status and future perspective. J Neurol Neurosurg Psychiatry. 2021;92:311–318. - PubMed
-
- Leonhard SE, van der Eijk AA, Andersen H, et al. An international perspective on preceding infections in Guillain-Barré syndrome: The IGOS-1000 cohort. Neurology. 2022;99:e1299–e1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

