Dynamic glucose enhanced imaging using direct water saturation
- PMID: 40096575
- PMCID: PMC12021318
- DOI: 10.1002/mrm.30447
Dynamic glucose enhanced imaging using direct water saturation
Abstract
Purpose: Dynamic glucose enhanced (DGE) MRI studies employ CEST or spin lock (CESL) to study glucose uptake. Currently, these methods are hampered by low effect size and sensitivity to motion. To overcome this, we propose to utilize exchange-based linewidth (LW) broadening of the direct water saturation (DS) curve of the water saturation spectrum (Z-spectrum) during and after glucose infusion (DS-DGE MRI).
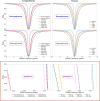
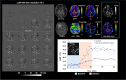
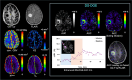
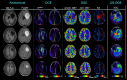
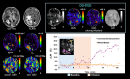
Methods: To estimate the glucose-infusion-induced LW changes (ΔLW), Bloch-McConnell simulations were performed for normoglycemia and hyperglycemia in blood, gray matter (GM), white matter (WM), CSF, and malignant tumor tissue. Whole-brain DS-DGE imaging was implemented at 3 T using dynamic Z-spectral acquisitions (1.2 s per offset frequency, 38 s per spectrum) and assessed on four brain tumor patients using infusion of 35 g of D-glucose. To assess ΔLW, a deep learning-based Lorentzian fitting approach was used on voxel-based DS spectra acquired before, during, and post-infusion. Area-under-the-curve (AUC) images, obtained from the dynamic ΔLW time curves, were compared qualitatively to perfusion-weighted imaging parametric maps.
Results: In simulations, ΔLW was 1.3%, 0.30%, 0.29/0.34%, 7.5%, and 13% in arterial blood, venous blood, GM/WM, malignant tumor tissue, and CSF, respectively. In vivo, ΔLW was approximately 1% in GM/WM, 5% to 20% for different tumor types, and 40% in CSF. The resulting DS-DGE AUC maps clearly outlined lesion areas.
Conclusions: DS-DGE MRI is highly promising for assessing D-glucose uptake. Initial results in brain tumor patients show high-quality AUC maps of glucose-induced line broadening and DGE-based lesion enhancement similar and/or complementary to perfusion-weighted imaging.
Keywords: CEST; Z‐spectra; direct saturation (DS); dynamic glucose enhanced (DGE) MRI; glucoCEST.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Under a license agreement between Philips and the Johns Hopkins University, L.K.'s spouse, P.C.M.v.Z., and the University are entitled to fees related to an imaging device used in the study discussed in this publication. P.C.M.v.Z. is also a paid lecturer for Philips. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
Figures

Update of
-
Dynamic glucose enhanced imaging using direct water saturation.ArXiv [Preprint]. 2025 Apr 24:arXiv:2410.17119v2. ArXiv. 2025. Update in: Magn Reson Med. 2025 Jul;94(1):15-27. doi: 10.1002/mrm.30447. PMID: 39502884 Free PMC article. Updated. Preprint.
References
-
- Mathur M, Jones JR, Weinreb JC. Gadolinium deposition and nephrogenic systemic fibrosis: a Radiologist's primer. Radiographics. 2020;40:153‐162. - PubMed
-
- Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium‐based contrast agent: a systematic review and meta‐analysis. JAMA Intern Med. 2020;180:223‐230. - PMC - PubMed
-
- Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. International Society for Magnetic Resonance in M. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564‐570. - PubMed
-
- McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast‐enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285:546‐554. - PubMed
-
- Lord ML, Chettle DR, Grafe JL, Noseworthy MD, McNeill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: results of a small pilot feasibility study. Radiology. 2018;287:96‐103. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

