Trans-Golgi network tethering factors regulate TBK1 trafficking and promote the STING-IFN-I pathway
- PMID: 40097395
- PMCID: PMC11914254
- DOI: 10.1038/s41421-024-00763-z
Trans-Golgi network tethering factors regulate TBK1 trafficking and promote the STING-IFN-I pathway
Abstract
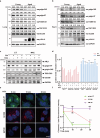
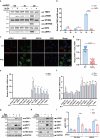
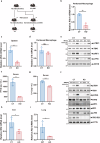
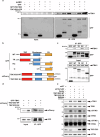
The cGAS-STING pathway mediates the innate immune response to cytosolic DNA, contributing to surveillance against microbial invasion or cellular damage. Once activated, STING recruits TBK1 at the trans-Golgi network (TGN), which in turn phosphorylates IRF3 to induce type I interferon (IFN-I) expression. In contrast to STING, little is known about how TBK1 is transported to the TGN for activation. Here, we show that multiple TGN tethering factors, a group of proteins involved in vesicle capturing, are indispensable for STING-IFN-I signaling. Deletion of TBC1D23, a recently reported tethering factor, in mice impairs the STING-IFN-I signaling, but with insignificant effect on STING-NF-κB signaling. Mechanistically, TBC1D23 interacts with TBK1 via the WASH complex subunit FAM21 and promotes its endosome-to-TGN translocation. Furthermore, multiple TGN tethering factors were reduced in aged mice and senescent fibroblasts. In summary, our study uncovers that TGN tethering factors are key regulators of the STING-IFN-I signaling and suggests that their reduction in senescence may produce aberrant STING signaling.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures

References
-
- Hopfner, K.-P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol.21, 501–521 (2020). - PubMed
-
- Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science347, aaa2630 (2015). - PubMed
-
- Fang, R. et al. NEMO–IKKβ Are Essential for IRF3 and NF-κB Activation in the cGAS–STING Pathway. J. Immunol.199, 3222–3233 (2017). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

