Monocyte-lineage tumor infiltration predicts immunoradiotherapy response in advanced pretreated soft-tissue sarcoma: phase 2 trial results
- PMID: 40097400
- PMCID: PMC11914280
- DOI: 10.1038/s41392-025-02173-3
Monocyte-lineage tumor infiltration predicts immunoradiotherapy response in advanced pretreated soft-tissue sarcoma: phase 2 trial results
Abstract
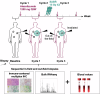
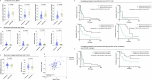
Immunoradiotherapy holds promise for improving outcomes in patients with advanced solid tumors, including in soft-tissue sarcoma (STS). However, the ideal combination of treatment modalities remains to be determined, and reliable biomarkers to predict which patients will benefit are lacking. Here, we report the results of the STS cohort of the SABR-PDL1 phase II trial that evaluated the anti-PDL1 atezolizumab combined with stereotactic body radiation therapy (SBRT) delivered concurrently with the 2nd cycle to at least one tumor site. Eligible patients received atezolizumab until progression or unmanageable toxicity, with SBRT at 45 Gy in 3 fractions). The primary endpoint was one-year progression-free survival (PFS) rate with success defined as 13 patients achieving 1-year PFS. Sixty-one heavily pretreated patients with STS (median 5 prior lines; 52% men; median age 54 years; 28% leiomyosarcoma) were enrolled across two centers (France, Spain). SBRT was delivered to 55 patients (90%), with the lung being the most commonly irradiated site (50%). After a median follow-up of 45 months, the one-year PFS rate was 8.3% [95% CI: 3.6-18.1]. Median PFS and overall survival were 2.5 and 8.6 months, respectively. Best responses included partial responses (5%) and stable disease (60%). Immune profiling revealed increased immunosuppressive tumor-associated macrophages (e.g., IL4I1, HES1) and monocyte-recruiting chemokines in non-responders. Higher monocyte/lymphocyte ratios (MonoLR) in tumor and blood correlated with progression. PD-L1 status, lymphoid infiltration, and tertiary-lymphoid structures were not predictive. Although the primary endpoint was not met, this study highlights MonoLR imbalance as a potential biomarker to identify STS patients likely to benefit from immunoradiotherapy. EudraCT No. 2015-005464-42; Clinicaltrial.gov number: NCT02992912.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.L. reports grants for academic research from PharMamar, Beigene, Amgen, Roche, and AstraZeneca outside of the submitted work. M.F. reports grants and personal fees from VIFOR Pharma and Pharmamar outside of the submitted work. M.M. reports grants from Boehringer Ingelheim outside of the submitted work. E.D. reports grants and personal fees from Roche-Genentech, AstraZeneca, Merck Serono, Boehringer, BMS, and MSD. R.S. declares research fundings from Daiichi Sankyo, Roche. and AstraZeneca outside of the submitted work. The other authors have nothing to declare.
Figures



References
-
- Gronchi, A. et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.32, 1348–1365 (2021). - PubMed
-
- Somaiah, N. et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single centre phase 2 trial. Lancet Oncol.23, 1156–1166 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

