Cortico-striatal circuit mechanisms drive the effects of D1 dopamine agonists on memory capacity in mice through cAMP/PKA signalling
- PMID: 40097401
- PMCID: PMC11914583
- DOI: 10.1038/s41467-025-57788-5
Cortico-striatal circuit mechanisms drive the effects of D1 dopamine agonists on memory capacity in mice through cAMP/PKA signalling
Abstract
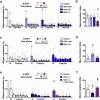
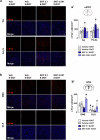
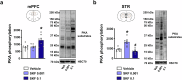
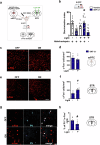
Working memory capacity (WMC), the number of items remembered in a short-time interval, is regulated by fronto-striatal dopamine (DA) and is reduced in schizophrenia. We investigated how excessive and insufficient D1 dopamine receptor stimulation impairs and expands WMC, focusing on the cAMP/PKA pathway in the fronto-striatal circuit. Low doses of the D1 agonist SKF 38393 enhance WMC by activating the striatum (mice remember more objects), while high doses, paradoxically, impair WMC, activating the same pathway in the medial prefrontal cortex (mPFC) but inhibiting it in the striatum. This impairment, arising from mPFC-driven recruitment of inhibitory striatal parvalbumin interneurons, can be prevented by optogenetic inhibition of the mPFC-striatal pathway. Low doses of SKF 38393 also rescue WMC deficits in a schizophrenia mouse model. These results highlight the need for a systems pharmacology approach that considers complex brain interactions and intracellular signalling pathways, rather than isolated drug-receptor interactions, to develop memory-enhancing treatments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Miller, G. A. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychol. Rev. 10.1037/0033-295x.101.2.343 (1994). - PubMed
-
- Sannino, S. et al. Role of the dorsal hippocampus in object memory load. Learn. Mem. 10.1101/lm.025213.111 (2012). - PubMed
-
- Fuster, J. M. & Alexander, G. E. Neuron activity related to short-term memory. Science173, 652–654 (1971). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

