A decrease in Flavonifractor plautii and its product, phytosphingosine, predisposes individuals with phlegm-dampness constitution to metabolic disorders
- PMID: 40097405
- PMCID: PMC11914097
- DOI: 10.1038/s41421-025-00789-x
A decrease in Flavonifractor plautii and its product, phytosphingosine, predisposes individuals with phlegm-dampness constitution to metabolic disorders
Abstract
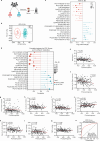
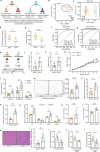
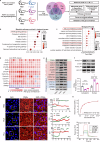
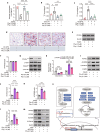
According to traditional Chinese medicine (TCM) constitutional theory, individuals with phlegm-dampness constitution (PDC) are at increased risk for metabolic disorders. Previous studies have indicated that PDC individuals exhibit gene expression changes associated with metabolic disorders, even individuals with normal metabolic indices. However, the biological mechanisms underlying these changes remain unclear. The gut microbiota has recently emerged as a promising avenue for elucidating TCM principles. Here, we revealed that individuals with PDC have distinct gut microbiota and serum metabolite profiles. A decrease in phytosphingosine was associated with increased PDC scores and metabolic disorder severity. Subsequent experiments demonstrated that Flavonifractor plautii can biosynthesize phytosphingosine, which was also negatively correlated with the PDC score. Interestingly, both F. plautii and phytosphingosine levels decreased in PDC subjects with normal metabolic indices. Fecal transplantation from these individuals accelerated the development of metabolic disorders in mice. However, supplementation with F. plautii and phytosphingosine ameliorated metabolic disorders by increasing phytosphingosine levels in the gut‒hepatic axis. Mechanistic investigations confirmed that phytosphingosine can directly bind to hepatic peroxisome proliferator-activated receptor α (PPARα) and activate its nuclear transcription activity, thereby regulating downstream gene expression related to glucose‒lipid metabolism. Our research indicates that the decrease in F. plautii and its product, phytosphingosine, contributes to gene expression changes related to metabolic disorders in PDC individuals and increases their susceptibility to metabolic disorders. These findings suggest that diagnosing PDC may be beneficial for identifying at-risk populations among apparently healthy individuals, thereby advancing the broader field of metabolic disorder prevention and TCM integration.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

