Measuring the effect of repetitive stretching on the deformability of human red blood cells using optical tweezers
- PMID: 40097548
- PMCID: PMC11914483
- DOI: 10.1038/s41598-025-93288-8
Measuring the effect of repetitive stretching on the deformability of human red blood cells using optical tweezers
Abstract
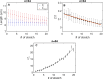
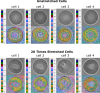
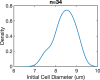
Mechanical features of cells play a crucial role in many biological processes such as crawling, proliferation, spreading, stretching, contracting, division, and programmed cell death. The loss of cell viscoelasticity underlines different types of diseases such as cancer, sickle cell, malaria, and diabetes mellitus. To understand the loss of viscoelasticity, mechanical responses of various kinds of cells to stress or strain are under investigation. Especially red blood cells (RBCs) or erythrocytes are one of the simple structured cells such that the effects of stress or strain could be easily assessed. With their viscoelastic nature, they can deform by preserving cell integrity when passing through blood vessels that are smaller than their size. In this study, we investigated the mechanical response of RBCs under repetitive stretching-relaxation cycles and examined some of the universal cytoskeleton laws at the single cell level over the whole body. For this, the individual RBCs were exposed to repetitive biaxial stretch-relaxation cycles of 5 s duration by optical tweezers to assess their mechanical response. According to the findings, the cells became stiffer with each stretch and became completely undeformable after a certain number of stretch-relaxation cycles. We observed that with the increasing number of stretching cycles, cell stiffness changed as a sign of weak power law, implying cell rheology is scale-free and decay times were increased, showing the transition from fast to slow regime. In addition, the appearance of the cells became non-uniform with darker areas in some parts and highly elongated shape in the most extreme cases.
Keywords: Cell mechanics; Cytoskeleton; Red blood cell.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
-
- Dao, M., Lim, C. T. & Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids51, 2259–2280 (2003).
-
- Lux, S. E. Anatomy of the red cell membrane skeleton: Unanswered questions. Blood127, 187–199 (2016). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

