Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial
- PMID: 40097657
- PMCID: PMC12003196
- DOI: 10.1038/s43018-025-00921-6
Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial
Erratum in
-
Author Correction: Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: primary efficacy and safety analysis in phase 2 ELM-2 trial.Nat Cancer. 2025 May;6(5):907. doi: 10.1038/s43018-025-00967-6. Nat Cancer. 2025. PMID: 40240622 Free PMC article. No abstract available.
Abstract
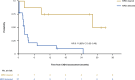
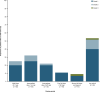
The phase 2, multicohort, ongoing ELM-2 study evaluates odronextamab, a CD20×CD3 bispecific antibody, in patients with relapsed/refractory (R/R) B cell non-Hodgkin lymphoma after ≥2 lines of therapy. Here primary analysis of the diffuse large B cell lymphoma (DLBCL) cohort is reported. Patients received intravenous odronextamab in 21-day cycles until progression or unacceptable toxicity, with cycle 1 step-up dosing to mitigate cytokine release syndrome (CRS) risk. The primary endpoint was objective response rate (ORR). Secondary endpoints included complete response (CR) rate, duration of response, progression-free survival (PFS) and overall survival. A total of 127 patients were enrolled. At the 29.9-month efficacy follow-up, the ORR was 52.0% and CR rate was 31.5%. Median durations of response and CR were 10.2 and 17.9 months, respectively. Undetectable minimal residual disease at cycle 4 day 15 was associated with PFS benefit. With a step-up of 0.7 to 4 to 20 mg (n = 60), CRS was the most common treatment-emergent adverse event (53.3% (grade ≥3, 1.7%)). No immune effector cell-associated neurotoxicity syndrome was reported. Infections were reported in 82/127 (64.6%) patients (grade ≥3, 38.6%; coronavirus disease 2019, 18.1% (grade ≥3, 12.6%)). In conclusion, odronextamab showed encouraging efficacy in heavily pretreated R/R DLBCL and generally manageable safety with supportive care. Clinical trial registration: NCT03888105 .
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: W.S.K. reports research funding from BeiGene, Boryung, Donga, Kyowa-Kirin, Roche and Sanofi. T.M.K. reports honoraria from Amgen, AstraZeneca, IMBDx, Janssen, MedImmune and Takeda, trial research funding to institution from AstraZeneca, consultancy for AstraZeneca, Boryung, F.Hoffmann-La Roche, Janssen, MedImmune, Novartis, Regeneron Pharmaceuticals, Inc., Roche, Samsung Bioepis, Takeda and Yuhan, speaker bureau fees for IMBDx, Janssen and Takeda, membership on an entity’s board of directors or advisory committees for BeiGene, Janssen, Novartis, Regeneron Pharmaceuticals, Inc., Roche and Takeda and an uncompensated relationship with AstraZeneca, Boryung, MedImmune, Novartis and Roche. I.J. reports consultancy for Amgen, AstraZeneca, Kyowa-Kirin, Novartis, Pfizer, Sobi and Takeda, research funding from Amgen, AstraZeneca, BeiGene, Incyte, Janssen, Regeneron Pharmaceuticals, Inc., Sobi and Takeda and honoraria fees from AstraZeneca, Incyte, Janssen, Novartis, Pfizer, Sobi and Takeda. E.I.-J. reports consultancy for AbbVie and AstraZeneca and honoraria from AbbVie, AstraZeneca, Janssen and Novartis. L.M.P. reports research funding from Regeneron Pharmaceuticals, Inc. B.T. reports honoraria from AbbVie, Gilead, Incyte and Kite. C.C. reports honoraria from Gilead, Novartis, Regeneron Pharmaceuticals, Inc. and Takeda, consultancy for BMS, Regeneron Pharmaceuticals, Inc. and Takeda and membership on an entity’s board of directors or advisory committees for Regeneron Pharmaceuticals, Inc. and Takeda. Sa.A. reports membership on an entity’s board of directors or advisory committee for AbbVie, AstraZeneca, BeiGene, Fate Therapeutics and Regeneron Pharmaceuticals, Inc., consultancy for ADC Therapeutics and AstraZeneca and speaker bureau fees from BeiGene and Genentech. J. Cai, M.U., S.S., J.B.-V., A.C., H.M. and S. Ambati hold stock or stock options for and are employees of Regeneron Pharmaceuticals, Inc. J.W. reports consultancy for AbbVie, Gilead, Novartis, Roche, Takeda and MSD, research or clinical trial funding from GSK, Novartis and Roche and honoraria from AbbVie, Amgen, Gilead, GSK, Novartis, Roche and Takeda. The other authors declare no competing interests.
Figures





References
-
- Duarte, C. & Kamdar, M. Management considerations for patients with primary refractory and early relapsed diffuse large B-cell lymphoma. Am. Soc. Clin. Oncol. Educ. Book43, e390802 (2023). - PubMed
-
- Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet396, 839–852 (2020). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

