Smooth muscle cell Piezo1 depletion results in impaired contractile properties in murine small bowel
- PMID: 40097724
- PMCID: PMC11914552
- DOI: 10.1038/s42003-025-07697-6
Smooth muscle cell Piezo1 depletion results in impaired contractile properties in murine small bowel
Abstract
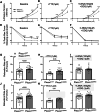
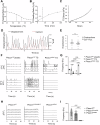
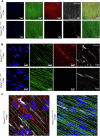
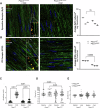
Piezo1 is a mechanosensitive cation channel expressed in intestinal muscularis cells (IMCs), including smooth muscle cells (SMCs), interstitial cells of Cajal, and Pdgfrα+ cells, which form the SIP syncytium, crucial for GI contractility. Here, we investigate the effects of SMC-specific Piezo1 deletion on small bowel function. Piezo1 depletion results in weight loss, delayed GI transit, muscularis thinning, and decreased SMCs. Ex vivo analyses demonstrated impaired contractile strength and tone, while in vitro studies using IMC co-cultures show dysrhythmic Ca2+ flux with decreased frequency. Imaging reveal that Piezo1 localizes intracellularly, thereby likely impacting Ca2+ signaling mechanisms modulated by Ca2 + -handling channels located on the sarcoplasmic reticulum and plasma membrane. Our findings suggest that Piezo1 in small bowel SMCs contributes to contractility by maintaining intracellular Ca2+ activity and subsequent signaling within the SIP syncytium. These findings provide new insights into the complex role of Piezo1 in small bowel SMCs and its implications for GI motility.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: None of the authors have any potential financial, professional, or personal conflicts.
Figures





References
-
- Mazet, B. Gastrointestinal motility and its enteric actors in mechanosensitivity: past and present. Pflügers Archiv: Eur. J. Physiol.467,191–200 (2014). - PubMed
-
- Furness, J. B, Kunze, W. A & Clerc, N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am. J. Physiol.277, G922–G928 (1999). - PubMed
-
- Holm, A. N., Rich, A., Sarr, M. G & Farrugia, G. Whole cell current and membrane potential regulation by a human smooth muscle mechanosensitive calcium channel. Am. J. Physiol. Gastrointest. Liver Physiol.279, G1155–G1161 (2000). - PubMed
-
- Costa, M. & Furness, J. B The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch. Pharm.294, 47–60 (1976). - PubMed
MeSH terms
Substances
Grants and funding
- L40 DK137356/DK/NIDDK NIH HHS/United States
- RC2 DK118640/DK/NIDDK NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- TB1-01183/California Institute for Regenerative Medicine (CIRM)
- P30 DK041301/DK/NIDDK NIH HHS/United States
- R01 DK083319/DK/NIDDK NIH HHS/United States
- KL2 TR001859/TR/NCATS NIH HHS/United States
- P30 DK41301/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- T32 DK007180/DK/NIDDK NIH HHS/United States
- Marshall Klaus 20202900/American Academy of Pediatrics (AAP)
- R01 DK083762/DK/NIDDK NIH HHS/United States
- R01 DK132319/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

