A Xanthine Derivative With Novel Heat Shock Protein 90-Alpha Inhibitory and Senolytic Properties
- PMID: 40098059
- PMCID: PMC12266748
- DOI: 10.1111/acel.70047
A Xanthine Derivative With Novel Heat Shock Protein 90-Alpha Inhibitory and Senolytic Properties
Abstract
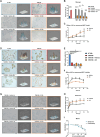
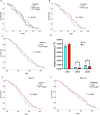
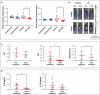
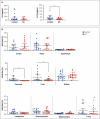
The accumulation of senescent cells contributes to aging and related diseases; therefore, discovering safe senolytic agents-compounds that selectively eliminate senescent cells-is a critical priority. Heat shock protein 90 (HSP90) inhibitors (HSP90i), traditionally investigated for cancer treatment, have shown potential as senolytic agents. However, inhibitors face formulation, toxicity, and cost challenges. To overcome these limitations, we employed a virtual screening approach combining structure-based prefiltering with a ligand-based pharmacophore model to identify novel, potentially safe HSP90 alpha isoform inhibitors exhibiting senolytic properties. This strategy identified 14 candidate molecules evaluated for senolytic activity in primary human fetal pulmonary fibroblasts. Four compounds exhibited significant HSP90i and senolytic activity, including two novel compounds, namely K4 and K5. The latter, 1-benzyl-3-(2-methylphenyl)-3,7-dihydro-1H-purine-2,6-dione, structurally related to the xanthinic family, emerged as a promising, well-tolerated senolytic agent. K5 demonstrated senolytic activity across various cellular senescence models, including human fibroblasts, mesenchymal stem cells, and breast cancer cells. It was also effective in vivo, extending lifespan in Drosophila and reducing senescence markers in geriatric mice. Additionally, the xanthinic nature of K5 implicates a multimodal action, now including the inhibition of HSP90α, that might enhance its efficacy and selectivity towards senescent cells, Senolytic index SI > 1320 for IMR90 cells, and SI > 770 for WI38 cells, underscoring its therapeutic potential. These findings advance senolytic therapy research, opening new avenues for safer interventions to combat age-related inflammaging and diseases, including cancer, and possibly extend a healthy lifespan.
Keywords: drosophila; HSP90α inhibitors; aging; senescence; senolytics; xanthine.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
All authors agreed to this publication.
Prof. Carlo Gaetano and Drs. Sandra Atlante, Maria Cristina De Rosa, Davide Pirolli, Antonella Farsetti, and Marco Malavolta declare a conflicts of interest as part of the data presented in this study was used to file a patent (WO 2023/135533 A1) which has subsequently been granted to INRCA, CNR, and ICS Maugeri.
Figures



References
-
- Abu‐Hashem, A. A. , Hakami O., El‐Shazly M., El‐Nashar H. A. S., and Yousif M. N. M.. 2024. “Caffeine and Purine Derivatives: A Comprehensive Review on the Chemistry, Biosynthetic Pathways, Synthesis‐Related Reactions, Biomedical Prospectives and Clinical Applications.” Chemistry & Biodiversity 21, no. 7: e202400050. 10.1002/cbdv.202400050. - DOI - PubMed
-
- Berglund, A. , De Rosa M. C., and Wold S.. 1997. “Alignment of Flexible Molecules at Their Receptor Site Using 3D Descriptors and Hi‐PCA.” Journal of Computer‐Aided Molecular Design 11: 601–612. - PubMed
-
- Brayton, C. F. , Treuting P. M., and Ward J. M.. 2012. “Pathobiology of Aging Mice and GEM: Background Strains and Experimental Design.” Veterinary Pathology 49: 85–105. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

