Inhaled Sedation in Acute Respiratory Distress Syndrome: The SESAR Randomized Clinical Trial
- PMID: 40098564
- PMCID: PMC11920880
- DOI: 10.1001/jama.2025.3169
Inhaled Sedation in Acute Respiratory Distress Syndrome: The SESAR Randomized Clinical Trial
Abstract
Importance: Whether the use of inhaled or intravenous sedation affects outcomes differentially in mechanically ventilated adults with acute respiratory distress syndrome (ARDS) is unknown.
Objective: To determine the efficacy and safety of inhaled sevoflurane compared with intravenous propofol for sedation in patients with ARDS.
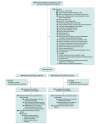
Design, setting, and participants: Phase 3 randomized, open-label, assessor-blinded clinical trial conducted from May 2020 to October 2023 with 90-day follow-up. Adults with early moderate to severe ARDS (defined by a ratio of Pao2 to the fraction of inspired oxygen of <150 mm Hg with a positive end-expiratory pressure of ≥8 cm H2O) were enrolled in 37 French intensive care units.
Interventions: Patients were randomized to a strategy of inhaled sedation with sevoflurane (intervention group) or to a strategy of intravenous sedation with propofol (control group) for up to 7 days.
Main outcomes and measures: The primary end point was the number of ventilator-free days at 28 days; the key secondary end point was 90-day survival.
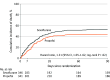
Results: Of 687 patients enrolled (mean [SD] age, 65 [12] years; 30% female), 346 were randomized to sevoflurane and 341 to propofol. The median total duration of sedation was 7 days (IQR, 4 to 7) in both groups. The number of ventilator-free days through day 28 was 0.0 days (IQR, 0.0 to 11.9) in the sevoflurane group and 0.0 days (IQR, 0.0 to 18.7) in the propofol group (median difference, -2.1 [95% CI, -3.6 to -0.7]; standardized hazard ratio, 0.76 [95% CI, 0.50 to 0.97]). The 90-day survival rates were 47.1% and 55.7% in the sevoflurane and propofol groups, respectively (hazard ratio, 1.31 [95% CI, 1.05 to 1.62]). Among 4 secondary outcomes, sevoflurane was associated with higher 7-day mortality (19.4% vs 13.5%, respectively; relative risk, 1.44 [95% CI, 1.02 to 2.03]) and fewer intensive care unit-free days through day 28 (median, 0.0 [IQR, 0.0 to 6.0] vs 0.0 [IQR, 0.0 to 15.0]; median difference, -2.5 [95% CI, -3.7 to -1.4]) compared with propofol.
Conclusions and relevance: Among patients with moderate to severe ARDS, inhaled sedation with sevoflurane resulted in fewer ventilator-free days at day 28 and lower 90-day survival than sedation with propofol.
Trial registration: ClinicalTrials.gov Identifier: NCT04235608.
Conflict of interest statement
Figures

Comment in
-
Sevoflurane Sedation in Acute Respiratory Distress Syndrome: Time to Put It to Sleep.JAMA. 2025 May 13;333(18):1586-1588. doi: 10.1001/jama.2025.3023. JAMA. 2025. PMID: 40098602 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

