Assessing zoonotic risk in a fenced natural park in northwestern Italy: integrating camera traps for a vector-host approach to investigate tick-borne pathogens
- PMID: 40098889
- PMCID: PMC11911494
- DOI: 10.3389/fvets.2025.1536260
Assessing zoonotic risk in a fenced natural park in northwestern Italy: integrating camera traps for a vector-host approach to investigate tick-borne pathogens
Abstract
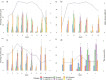
Tick-borne diseases are among the major widespread emerging zoonotic diseases, and their circulation in the environment is influenced by a broad range of abiotic and biotic factors, including the abundance of vectors and vertebrate hosts. In this study, we estimated the prevalence of tick-borne pathogens and the impact of wildlife head count on their circulation in a lowland natural area in northwestern Italy. We collected ticks and camera trap pictures from 14 sampling points every 2 weeks for 1 year and identified pathogens through molecular analyses: Babesia capreoli, B. microti-like, Borrelia burgdorferi sensu lato (s.l.), Rickettsia of the spotted fever group (SFG), Theileria capreoli, and Anaplasma phagocytophilum. We modeled the presence of B. capreoli, B. microti-like, B. burgdorferi s.l., and SFG Rickettsia on head counts of wild ungulates and mesocarnivores. We tested a global model including all collected ticks, as well as a model focusing solely on Ixodes ricinus nymphs, the species, and the developmental stage most associated with zoonotic infection risk. The highest prevalence was obtained for B. microti-like (13%) and SFG Rickettsia (11%), and, for most pathogens, no differences were detected among tick species and their developmental stages. Mesocarnivores showed an additive effect on B. microti-like and B. burgdorferi s.l., while wild ungulates, non-competent for transmission of our target pathogens, showed a dilutive effect. These findings confirm the circulation of relevant tick-borne pathogens in the study area and show the use of camera trap data in predicting tick-borne pathogens' risk by targeting host species which may have an indirect impact and are more easily addressed by monitoring and control strategies.
Keywords: Ixodidae; humans; recreational areas; tick-borne zoonoses; wildlife.
Copyright © 2025 Vada, Zanet, Occhibove, Trisciuoglio, Varzandi and Ferroglio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
The potential risk of human exposure to tick borne infection by Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, and Babesia microti in selected recreational areas of the Poprad Landscape Park in southern Poland.Ann Agric Environ Med. 2024 Sep 25;31(3):345-350. doi: 10.26444/aaem/186025. Epub 2024 Mar 27. Ann Agric Environ Med. 2024. PMID: 39344722
-
Ticks are more suitable than red foxes for monitoring zoonotic tick-borne pathogens in northeastern Italy.Parasit Vectors. 2018 Mar 20;11(1):137. doi: 10.1186/s13071-018-2726-7. Parasit Vectors. 2018. PMID: 29554970 Free PMC article.
-
Prevalence of tick-borne pathogens in ticks collected from the wild mountain ungulates mouflon and chamois in 4 regions of France.Parasite. 2024;31:21. doi: 10.1051/parasite/2024011. Epub 2024 Apr 10. Parasite. 2024. PMID: 38602373 Free PMC article.
-
[Ixodes ricinus, transmitted diseases and reservoirs].Parassitologia. 2004 Jun;46(1-2):119-22. Parassitologia. 2004. PMID: 15305699 Review. Italian.
-
Ticks and their epidemiological role in Slovakia: from the past till present.Biologia (Bratisl). 2022;77(6):1575-1610. doi: 10.1007/s11756-021-00845-3. Epub 2021 Sep 17. Biologia (Bratisl). 2022. PMID: 34548672 Free PMC article. Review.
References
-
- European Centre for Disease Prevention and Control . Communicable disease threats report, 2–8 August 2020, week 32. Stockholm: European Centre for Disease Prevention and Control; (2020).
LinkOut - more resources
Full Text Sources

