A Split-Face Micro-Needling Study to Evaluate the Efficacy and Consumer Perception of a Novel Moisturization Agent
- PMID: 40099382
- PMCID: PMC11915079
- DOI: 10.1111/jocd.70109
A Split-Face Micro-Needling Study to Evaluate the Efficacy and Consumer Perception of a Novel Moisturization Agent
Abstract
Background: Wound healing is essential for restoring skin integrity following damage. The skin barrier plays a critical role in protecting against infection, preventing moisture loss, and supporting regeneration. Ceramides, integral components of the lipid matrix, are known to improve skin hydration, reduce inflammation, and accelerate wound healing. However, research on ceramide-based formulations in post-procedural settings remains limited.
Aims: This study aims to evaluate the efficacy of Aestura ATOBARRIER 365 Cream, containing a Lipid Complex with ceramides, cholesterol, and fatty acids, in promoting skin barrier recovery and improving outcomes after barrier disruption.
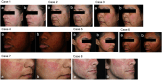
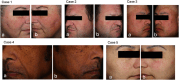
Methods: A randomized, double-blind, split-face trial was conducted with 30 participants aged 22-60 years. Following microneedling, the active formulation was applied to one side of the face and the vehicle formulation to the other, twice daily for 4 weeks. Transepidermal Water Loss (TEWL), erythema, and roughness were measured at baseline, post-application, and at weeks 2 and 4. Participant surveys assessed moisturization, erythema, and overall skin improvement.
Results: Both formulations demonstrated significant reductions in TEWL (14%-16%) and erythema (~1.7%) by week 4 compared to post-microneedling. High participant satisfaction was also observed, with 93% reporting improved adequate skin hydration and 90% reporting reduced erythema with the active formulation.
Conclusions: Aestura ATOBARRIER 365 Cream with ceramides demonstrated efficacy in improving skin barrier recovery, reducing TEWL, and enhancing skin texture following microneedling. The significant reductions in TEWL, erythema, and roughness highlight its ability to restore the skin's barrier, calm irritation, and refine texture. These findings confirm its role in supporting skin recovery and resilience after dermatological treatments, making it a valuable addition to post-procedure care. While these results support its effectiveness in post-procedural recovery, further research is needed to determine its applicability for other conditions involving compromised skin barriers, such as eczema or rosacea. Additional studies are also warranted to assess long-term efficacy and its potential role in optimizing skin barrier restoration across diverse patient populations.
Keywords: Transepidermal water loss; ceramides; skin barrier; wound healing.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Jane Y. Yoo serves as a consultant for Amorepacific US Inc. Jinseob Shin and Michelle Shieh are employees of Amorepacific US Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

