Size-Dependent Target Engagement of Covalent Probes
- PMID: 40099438
- PMCID: PMC11956015
- DOI: 10.1021/acs.jmedchem.5c00017
Size-Dependent Target Engagement of Covalent Probes
Abstract
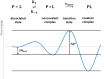
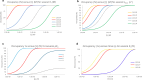
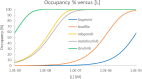
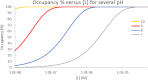
Labeling proteins with covalent ligands is finding increasing use in proteomics applications, including identifying nucleophilic residues amenable for labeling and in the development of targeted covalent inhibitors (TCIs). Labeling efficiency is measured by the covalent occupancy of the target or by biochemical activity. Here, we investigate how these observed quantities relate to the intrinsic parameters of complex formation, namely, noncovalent affinity and covalent reactivity, and to experimental conditions, including incubation time and ligand concentration. It is shown that target engagement is beneficially driven by noncovalent recognition for lead-like compounds, which are appropriate starting points for targeted covalent inhibitors owing to their easily detectable occupancy and fixed binding mode, facilitating optimization. In contrast, labeling by fragment-sized compounds is inevitably reactivity-driven as their small size limits noncovalent affinity. They are well-suited for exploring ligandable nucleophilic residues, while small fragments are less appropriate starting points for TCI development.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Recent progress in covalent warheads for in vivo targeting of endogenous proteins.Bioorg Med Chem. 2021 Oct 1;47:116386. doi: 10.1016/j.bmc.2021.116386. Epub 2021 Aug 27. Bioorg Med Chem. 2021. PMID: 34509863 Review.
-
Phosphatidylinositol 3,4,5-trisphosphate activity probes for the labeling and proteomic characterization of protein binding partners.Biochemistry. 2011 Dec 27;50(51):11143-61. doi: 10.1021/bi201636s. Epub 2011 Nov 30. Biochemistry. 2011. PMID: 22074223 Free PMC article.
-
Modeling the Binding and Conformational Energetics of a Targeted Covalent Inhibitor to Bruton's Tyrosine Kinase.J Chem Inf Model. 2021 Oct 25;61(10):5234-5242. doi: 10.1021/acs.jcim.1c00897. Epub 2021 Sep 30. J Chem Inf Model. 2021. PMID: 34590480
-
Peptide-Based Covalent Inhibitors Bearing Mild Electrophiles to Target a Conserved His Residue of the Bacterial Sliding Clamp.JACS Au. 2024 Jan 25;4(2):432-440. doi: 10.1021/jacsau.3c00572. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425897 Free PMC article.
-
N-Acyl-N-alkyl/aryl Sulfonamide Chemistry Assisted by Proximity for Modification and Covalent Inhibition of Endogenous Proteins in Living Systems.Acc Chem Res. 2025 Jan 7;58(1):87-100. doi: 10.1021/acs.accounts.4c00628. Epub 2024 Dec 11. Acc Chem Res. 2025. PMID: 39661110 Review.
References
-
- Resnick E.; Bradley A.; Gan J.; Douangamath A.; Krojer T.; Sethi R.; Geurink P. P.; Aimon A.; Amitai G.; Bellini D.; Bennett J.; Fairhead M.; Fedorov O.; Gabizon R.; Gan J.; Guo J.; Plotnikov A.; Reznik N.; Ruda G. F.; Díaz-Sáez L.; Straub V. M.; Szommer T.; Velupillai S.; Zaidman D.; Zhang Y.; Coker A. R.; Dowson C. G.; Barr H. M.; Wang C.; Huber K. V. M.; Brennan P. E.; Ovaa H.; Von Delft F.; London N. Rapid Covalent-Probe Discovery by Electrophile-Fragment Screening. J. Am. Chem. Soc. 2019, 141 (22), 8951–8968. 10.1021/jacs.9b02822. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

