Olaparib Plus Abiraterone in Asian Patients With Metastatic Castration-Resistant Prostate Cancer: PROpel Subset Analysis
- PMID: 40099663
- PMCID: PMC12127112
- DOI: 10.1111/cas.16459
Olaparib Plus Abiraterone in Asian Patients With Metastatic Castration-Resistant Prostate Cancer: PROpel Subset Analysis
Abstract
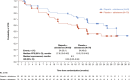
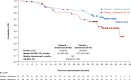
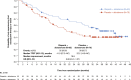
In the phase 3 PROpel trial (NCT03732820) patients with metastatic castration-resistant prostate cancer (mCRPC) treated with olaparib plus abiraterone in the first-line setting showed significantly prolonged radiographic progression-free survival (rPFS; primary data cutoff [DCO]: 30 July 2021; hazard ratio [HR] 0.66, 95% confidence interval [CI], 0.54-0.81; p < 0.001), and at prespecified final OS analysis DCO (12 October 2022) numerically prolonged overall survival (OS; HR 0.81, 95% CI, 0.67-1.00; p = 0.054), versus placebo plus abiraterone for the global population. Here, we report efficacy, safety, and patient-reported outcome data for the Asian subset in PROpel. Eligible patients were randomly assigned (1:1) to either olaparib (300 mg twice daily) or placebo in combination with abiraterone (1000 mg once daily). The primary endpoint was investigator-assessed rPFS, and a key secondary endpoint was OS. In the Asian subset (n = 133) at primary analysis, median rPFS was 27.6 months in the olaparib plus abiraterone arm (n = 63), compared with 19.3 months in the placebo plus abiraterone arm (n = 70; HR 0.55, 95% CI, 0.32-0.95). Median OS at the final analysis was not reached in the olaparib plus abiraterone arm versus 43.7 months in the placebo plus abiraterone arm (HR 0.59, 95% CI, 0.32-1.06). The safety profile was generally similar in the Asian subset and the global population. Efficacy and safety results for olaparib plus abiraterone in the Asian subset were generally consistent with the global PROpel population supporting the combination of olaparib plus abiraterone as an important first-line treatment for consideration in Asian patients with mCRPC. Trial Registration: Clinicaltrials.gov identifier: NCT03732820.
Keywords: Asian; abiraterone; metastatic castration‐resistant prostate cancer; olaparib; progression‐free survival.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Figures
References
-
- Ryan C. J., Smith M. R., Fizazi K., et al., “Abiraterone Acetate Plus Prednisone Versus Placebo Plus Prednisone in Chemotherapy‐Naive Men With Metastatic Castration‐Resistant Prostate Cancer (COU‐AA‐302): Final Overall Survival Analysis of a Randomised, Double‐Blind, Placebo‐Controlled Phase 3 Study,” Lancet Oncology 16 (2015): 152–160. - PubMed
-
- Armstrong A. J., Lin P., Tombal B., et al., “Five‐Year Survival Prediction and Safety Outcomes With Enzalutamide in Men With Chemotherapy‐Naive Metastatic Castration‐Resistant Prostate Cancer From the PREVAIL Trial,” European Urology 78 (2020): 347–357. - PubMed
-
- Sayegh N., Swami U., and Agarwal N., “Recent Advances in the Management of Metastatic Prostate Cancer,” JCO Oncology Practice 18 (2021): 45–55. - PubMed
-
- Clarke N., Wiechno P., Alekseev B., et al., “Olaparib Combined With Abiraterone in Patients With Metastatic Castration‐Resistant Prostate Cancer: A Randomised, Double‐Blind, Placebo‐Controlled, Phase 2 Trial,” Lancet Oncology 19 (2018): 975–986. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

