Changes in Adrenal Function and Insufficiency Symptoms After Cessation of Prednisolone
- PMID: 40100216
- PMCID: PMC11920843
- DOI: 10.1001/jamanetworkopen.2025.1029
Changes in Adrenal Function and Insufficiency Symptoms After Cessation of Prednisolone
Abstract
Importance: The widespread use of glucocorticoid (GC) therapy may result in GC-induced adrenal insufficiency (GIAI), but the prevalence and clinical implications remain uncertain.
Objective: To ascertain the prevalence and symptoms of GIAI.
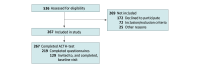
Design, setting, and participants: Cross-sectional multicenter study at 3 Danish hospitals. Baseline data were collected March 2021 to March 2024 from an ongoing randomized clinical trial. Participants were patients with polymyalgia rheumatica and/or giant cell arteritis who were investigated a median (IQR) of 39 (25-62) days after planned cessation of prednisolone treatment.
Exposure: Prednisolone treatment a median (IQR) of 13 (10-20) months in duration.
Main outcomes and measures: Primary outcome GIAI was defined as a stimulated plasma cortisol level less than 420 nmol/L in response to a short 250 μg corticotropin test (SST). Secondary outcomes were adrenal insufficiency symptoms assessed by the Addison disease-specific quality of life questionnaire (AddiQoL-30), body composition, and muscle function.
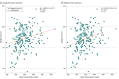
Results: Of 267 patients included (145 female [55%]; median [IQR] age 73 [68-78] years), 5 (1.9%; 95% CI, 0.8%-4.3%) had GIAI, whereas 75 (34%; 95% CI, 28%-41%) had symptoms compatible with adrenal insufficiency defined by an AddiQoL-30 score 85 or lower (symptomatic group). The symptomatic group had lower basal cortisol levels compared with the asymptomatic group (263 nmol/L; 95% CI, 242-283 nmol/L vs 309 nmol/L; 95% CI, 295-324 nmol/L; P < .001). Factors associated with a low AddiQoL-30 score included female sex (prevalence ratio [PR], 1.68; 95% CI, 1.13-2.51), increased body fat percentage (PR, 2.33; 95% CI, 1.21-4.50), reduced handgrip strength (PR, 2.71; 95% CI, 1.44-5.10) and low Short Physical Performance Battery score (PR, 2.78; 95% CI, 1.42-5.42).
Conclusions and relevance: This cross-sectional study of 267 patients with polymyalgia rheumatica or giant cell arteritis found a GIAI prevalence of 1.9% after cessation of prednisolone. This is much lower than previously reported and speaks against routine screening, which should be restricted to patients with overt symptoms. The high prevalence of symptoms of adrenal insufficiency in association with lower basal cortisol levels substantiate the clinical challenges of steroid withdrawal and merit future research.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

