Pain persists in mice lacking both Substance P and CGRPα signaling
- PMID: 40100256
- PMCID: PMC11919252
- DOI: 10.7554/eLife.93754
Pain persists in mice lacking both Substance P and CGRPα signaling
Abstract
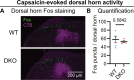
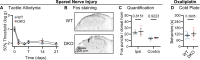
The neuropeptides Substance P and CGRPα have long been thought important for pain sensation. Both peptides and their receptors are expressed at high levels in pain-responsive neurons from the periphery to the brain making them attractive therapeutic targets. However, drugs targeting these pathways individually did not relieve pain in clinical trials. Since Substance P and CGRPα are extensively co-expressed, we hypothesized that their simultaneous inhibition would be required for effective analgesia. We therefore generated Tac1 and Calca double knockout (DKO) mice and assessed their behavior using a wide range of pain-relevant assays. As expected, Substance P and CGRPα peptides were undetectable throughout the nervous system of DKO mice. To our surprise, these animals displayed largely intact responses to mechanical, thermal, chemical, and visceral pain stimuli, as well as itch. Moreover, chronic inflammatory pain and neurogenic inflammation were unaffected by loss of the two peptides. Finally, neuropathic pain evoked by nerve injury or chemotherapy treatment was also preserved in peptide-deficient mice. Thus, our results demonstrate that even in combination, Substance P and CGRPα are not required for the transmission of acute and chronic pain.
Keywords: mouse; neuropeptides; neuroscience; pain; somatosensation.
Conflict of interest statement
DM, MJ, JS, RB, AN No competing interests declared, AC Reviewing editor, eLife
Figures




Update of
-
Pain persists in mice lacking both Substance P and CGRPα signaling.bioRxiv [Preprint]. 2024 Dec 2:2023.11.15.567208. doi: 10.1101/2023.11.15.567208. bioRxiv. 2024. Update in: Elife. 2025 Mar 18;13:RP93754. doi: 10.7554/eLife.93754. PMID: 38076807 Free PMC article. Updated. Preprint.
References
-
- Bohic M, Pattison LA, Jhumka ZA, Rossi H, Thackray JK, Ricci M, Mossazghi N, Foster W, Ogundare S, Twomey CR, Hilton H, Arnold J, Tischfield MA, Yttri EA, St John Smith E, Abdus-Saboor I, Abraira VE. Mapping the neuroethological signatures of pain, analgesia, and recovery in mice. Neuron. 2023;111:2811–2830. doi: 10.1016/j.neuron.2023.06.008. - DOI - PMC - PubMed
-
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt S-E. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. PNAS. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

