The autophagy protein ATG14 safeguards against unscheduled pyroptosis activation to enable embryo transport during early pregnancy
- PMID: 40100261
- PMCID: PMC11919251
- DOI: 10.7554/eLife.97325
The autophagy protein ATG14 safeguards against unscheduled pyroptosis activation to enable embryo transport during early pregnancy
Abstract
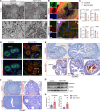
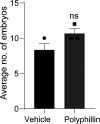
Recurrent pregnancy loss, characterized by two or more failed clinical pregnancies, poses a significant challenge to reproductive health. In addition to embryo quality and endometrial function, proper oviduct function is also essential for successful pregnancy establishment. Therefore, structural abnormalities or inflammation resulting from infection in the oviduct may impede the transport of embryos to the endometrium, thereby increasing the risk of miscarriage. However, our understanding of the biological processes that preserve the oviductal cellular structure and functional integrity is limited. Here, we report that autophagy-related protein ATG14 plays a crucial role in maintaining the cellular integrity of the oviduct by controlling inflammatory responses, thereby supporting efficient embryo transport. Specifically, the conditional depletion of the autophagy-related gene Atg14 in the oviduct causes severe structural abnormalities compromising its cellular integrity, leading to the abnormal retention of embryos. Interestingly, the selective loss of Atg14 in oviduct ciliary epithelial cells did not impact female fertility, highlighting the specificity of ATG14 function in distinct cell types within the oviduct. Mechanistically, loss of Atg14 triggered unscheduled pyroptosis via altering the mitochondrial integrity, leading to inappropriate embryo retention and impeded embryo transport in the oviduct. Finally, pharmacological activation of pyroptosis in pregnant mice phenocopied the genetically induced defect and caused impairment in embryo transport. Together, we found that ATG14 safeguards against unscheduled pyroptosis activation to enable embryo transport from the oviduct to uterus for the successful implantation. Of clinical significance, these findings provide possible insights into the underlying mechanism(s) of early pregnancy loss and might aid in developing novel prevention strategies using autophagy modulators.
Keywords: Atg14; autophagy; developmental biology; inflammation; mouse; oviduct; pregnancy.
© 2024, Popli et al.
Conflict of interest statement
PP, AO, VM, MR, YZ, MH, RM, JL, SA, KM, RK No competing interests declared
Figures









Update of
-
The autophagy protein, ATG14 safeguards against unscheduled pyroptosis activation to enable embryo transport during early pregnancy.bioRxiv [Preprint]. 2024 Nov 25:2024.03.19.585812. doi: 10.1101/2024.03.19.585812. bioRxiv. 2024. Update in: Elife. 2025 Mar 18;13:RP97325. doi: 10.7554/eLife.97325. PMID: 38562843 Free PMC article. Updated. Preprint.
References
-
- Chojnacki AK, Navaneetha Krishnan S, Jijon H, Shutt TE, Colarusso P, McKay DM. Tissue imaging reveals disruption of epithelial mitochondrial networks and loss of mitochondria-associated cytochrome-C in inflamed human and murine colon. Mitochondrion. 2023;68:44–59. doi: 10.1016/j.mito.2022.10.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R01HD104813/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R35HL145242/HL/NHLBI NIH HHS/United States
- R01HD065435/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R01 HD104813/HD/NICHD NIH HHS/United States
- R01 HD102680/HD/NICHD NIH HHS/United States
- R01 HD042311/HD/NICHD NIH HHS/United States
- R01HD102680/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R35 HL145242/HL/NHLBI NIH HHS/United States
- R01 HD065435/HD/NICHD NIH HHS/United States
- R01 HD-042311/Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources

