Mistargeted retinal axons induce a synaptically independent subcircuit in the visual thalamus of albino mice
- PMID: 40100272
- PMCID: PMC11919250
- DOI: 10.7554/eLife.100990
Mistargeted retinal axons induce a synaptically independent subcircuit in the visual thalamus of albino mice
Abstract
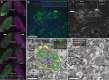
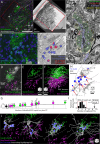
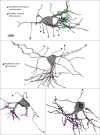
In albino mice and EphB1 knockout mice, mistargeted retinal ganglion cell axons form dense islands of axon terminals in the dorsal lateral geniculate nuclei (dLGN). The formation of these islands of retinal input depends on developmental patterns of spontaneous retinal activity. We reconstructed the microcircuitry of the activity-dependent islands and found that the boundaries of the island represent a remarkably strong segregation within retinogeniculate connectivity. We conclude that when sets of retinal input are established in the wrong part of the dLGN, the developing circuitry responds by forming a synaptically isolated subcircuit within the otherwise fully connected network. The fact that there is a developmental starting condition that can induce a synaptically segregated microcircuit has important implications for our understanding of the organization of visual circuits and our understanding of the implementation of activity-dependent development.
Keywords: Hebbian; albino; lateral geniculate nucleus; mouse; neuroscience; retinal ganglion cell; visual development.
© 2024, McCracken et al.
Conflict of interest statement
SM, LM, ZH, JH, KV, PW, JM No competing interests declared
Figures



Update of
-
Mistargeted retinal axons induce a synaptically independent subcircuit in the visual thalamus of albino mice.bioRxiv [Preprint]. 2024 Dec 3:2024.07.15.603571. doi: 10.1101/2024.07.15.603571. bioRxiv. 2024. Update in: Elife. 2025 Mar 18;13:RP100990. doi: 10.7554/eLife.100990. PMID: 39071408 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

