No operation after short-course radiotherapy followed by consolidation chemotherapy in locally advanced rectal cancer (NOAHS-ARC): study protocol for a prospective, phase II trial
- PMID: 40100473
- PMCID: PMC11919929
- DOI: 10.1007/s00384-025-04850-9
No operation after short-course radiotherapy followed by consolidation chemotherapy in locally advanced rectal cancer (NOAHS-ARC): study protocol for a prospective, phase II trial
Abstract
Purpose: Organ preservation through a watch-and-wait (W&W) strategy has become a viable option for select rectal cancer patients with clinical complete responses (cCR) to total neoadjuvant therapy (TNT). This approach limits the morbidity associated with multimodal treatment. However, the optimal treatment strategy and predictors of treatment response are still unresolved. Rectal cancer incidence is rising, particularly in developing countries, and the disease is a major public health concern in Chile. Prior to the no operation after short-course radiotherapy followed by consolidation chemotherapy in locally advanced rectal cancer (NOAHS-ARC) trial, TNT-based treatments and W&W programs had not been implemented in Chile.
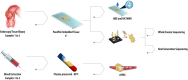
Methods/design: This single-arm, multicenter, phase II prospective trial, conducted in Santiago, Chile, will enroll patients with stage II/III rectal adenocarcinoma. Treatment involves induction short-course radiotherapy (25 Gy in 5 fractions) followed by consolidation chemotherapy (FOLFOX × 9 or CAPOX × 6 cycles). The response will be assessed 4-8 weeks after chemotherapy completion. Patients achieving cCR will be offered W&W, while those with incomplete responses will undergo total mesorectal excision. The primary endpoint is the rate of complete tumor response, combining pathologic complete responses (pCR) and sustained cCR (> 1 year), compared to a matched cohort treated with neoadjuvant chemoradiation alone. The trial aims to recruit 48 patients, assuming a combined pCR/sustained cCR rate of 12%. Quality of life measures will be assessed, and a biorepository of tissue and plasma samples will be established for future research, alongside serial endoscopic and MRI images.
Discussion: NOAHS-ARC seeks to advance organ preservation strategies in rectal cancer while pioneering TNT and W&W protocols in Chile. The study will also focus on functional outcomes and provide valuable data for improving patient care both locally and globally.
Trial registration: ClinicalTrials.gov identifier NCT04864067. Registered on April 28, 2021.
Keywords: Nonoperative management; Organ preservation; Short-course radiotherapy; Total neoadjuvant therapy; Watch-and-wait.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests.
Figures
References
-
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 - PubMed
-
- Arnold M, Sierra M, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691. 10.1136/gutjnl-2015-310912 - PubMed
-
- Sierra MS, Forman D (2016) Burden of colorectal cancer in Central and South America. Cancer Epidemiol 44(1):S74–S81. 10.1016/j.canep.2016.03.010 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous