Targeting malignant adenomyoepithelioma of the breast: clinical insights on multimodal therapy and disease-free survival
- PMID: 40100534
- PMCID: PMC11920443
- DOI: 10.1007/s12672-025-02120-2
Targeting malignant adenomyoepithelioma of the breast: clinical insights on multimodal therapy and disease-free survival
Abstract
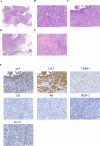
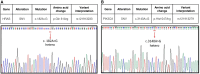
Adenomyoepithelioma of the breast (AME) is a rare, biphasic tumor characterized by the coexistence of both epithelial and myoepithelial cell components, which can present as benign, atypical, or malignant forms. We present a 50-year-old female diagnosed with malignant AME (M-AME) exhibiting low-positive estrogen receptor (ER) expression of 5%, alongside pathogenic HRAS Q61R and PIK3CA H1047R mutations. Immunohistochemistry showed low-positive ER, negative progesterone receptor (PR), HER2, and a high Ki-67 index. Sanger sequencing identified HRAS and PIK3CA mutations. The tumor was staged as pT2N0M0 with no lymph node involvement. The patient underwent partial mastectomy, followed by sentinel lymph node biopsy, which showed no metastasis. Postoperatively, she received four cycles of adjuvant chemotherapy, followed by radiotherapy. The patient achieved disease-free survival at 10 months with no recurrence on imaging. This case highlights the challenges of ER classification in M-AME and highlights the significance of molecular profiling in guiding treatment. The concurrent HRAS and PIK3CA mutations suggest potential targeted therapies, emphasizing the importance of a multidisciplinary approach. Further research is needed to establish standardized treatment guidelines for M-AME.
Keywords: Adenomyoepithelioma; Estrogen receptor; HRAS Q61; PIK3CA; Sanger sequencing.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was reviewed and approved by the Ethics Committee of Chung Shan Medical University Hospital. The research adhered to the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient. All procedures followed relevant guidelines and regulations to ensure ethical compliance. Competing interests: The authors declare no competing interests.
Figures
References
-
- Rakha E, Tan PH, Ellis I, Quinn C. Adenomyoepithelioma of the breast: a proposal for classification. Histopathology. 2021;79:465–79. - PubMed
-
- Nadelman CM, Leslie KO, Fishbein MC. “Benign,” metastasizing adenomyoepithelioma of the breast: a report of 2 cases. Arch Pathol Lab Med. 2006;130:1349–53. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
