Reliability of an all-in-one wearable sensor for continuous vital signs monitoring in high-risk patients: the NIGHTINGALE clinical validation study
- PMID: 40100556
- PMCID: PMC12474673
- DOI: 10.1007/s10877-025-01279-x
Reliability of an all-in-one wearable sensor for continuous vital signs monitoring in high-risk patients: the NIGHTINGALE clinical validation study
Abstract
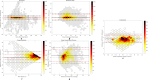
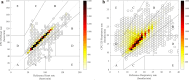
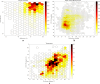
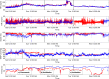
Continuous vital signs monitoring with wearable systems may improve early recognition of patient deterioration on hospital wards. The objective of this study was to determine whether the wearable Checkpoint Cardio's CPC12S, can accurately measure heart rate (HR), respiratory rate (RR), oxygen saturation (SpO2), blood pressure (BP) and temperature continuously. In an observational multicenter method comparison study of 70 high-risk surgical patients admitted to high-dependency wards; HR, RR, SpO2, BP and temperature were simultaneously measured with the CPC12S system and with ICU-grade monitoring systems in four European hospitals. Outcome measures were bias and 95% limits of agreement (LoA). Clinical accuracy was assessed with Clarke Error Grid analyses for HR and RR. A total of 3,212 h of vital signs data (on average 26 h per patient) were analyzed. For HR, bias (95% LoA) of the pooled analysis was 0.0 (-3.5 to 3.4), for RR 1.5 (-3.7 to 7.5) and for SpO2 0.4 (-3.1 to 4.0). The CPC12S system overestimated BP, with a bias of 8.9 and wide LoA (-23.3 to 41.2). Temperature was underestimated with a bias of -0.6 and LoA of -1.7 to 0.6. Clarke Error Grid analyses showed that adequate treatment decisions regarding changes in HR and RR would have been made in 99.2% and 92.0% of cases respectively. The CPC12S system showed high accuracy for measurements of HR. The accuracy of RR, SpO2 were slightly overestimated and core temperature underestimated, with LoA outside the predefined clinical acceptable range. The accuracy of BP was unacceptably low.
Keywords: Clinical deterioration; Continuous monitoring; Remote monitoring; Vital signs; Wearable device.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of each of the Medical Research Ethics Committees of the University Medical Center Utrecht (No. 20/078), Karolinksa University Hospital (No. 2020–04537), University Hospital Aachen (No. EK 417/20) and University Hospitals Leuven (No. B3222020000163). Consent to participate: Informed consent was obtained from all individidual participants included in the study. Competing interests: The authors declare no competing interests.
Figures
References
-
- Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL et al. Antecedents to hospital deaths. Intern Med J [Internet]. 2001;31:343–8. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1445-5994.2001.00077.x.... - DOI - PubMed
-
- Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest [Internet]. 1990;98:1388–92. Available from: https://journal.chestnet.org/article/S0012-3692(16)40939-6/fulltext - PubMed
-
- Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation [Internet]. 2004;62:137–41. Available from: https://www.resuscitationjournal.com/article/S0300-9572(04)00123-6/fulltext - PubMed
-
- Hillman KM, Bristow PJ, Chey T, Daffurn K, Jacques T, Norman SL, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Med. 2002;28:1629–34. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

