Genetics of primary congenital hypothyroidism: three decades of discoveries and persisting etiological challenges
- PMID: 40100854
- PMCID: PMC12002738
- DOI: 10.1530/ETJ-24-0348
Genetics of primary congenital hypothyroidism: three decades of discoveries and persisting etiological challenges
Abstract
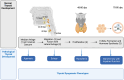
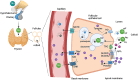
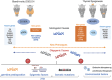
Primary congenital hypothyroidism (CH) is the most common neonatal endocrine disorder, and may be etiologically subdivided into thyroid dysgenesis, referring to abnormal thyroid development, and dyshormonogenesis, where a defective thyroid hormone biosynthesis pathway results in inadequate hormone production despite a structurally intact gland. Delayed treatment of neonatal hypothyroidism may result in irreversible neurodevelopmental impairment; therefore, where available, CH screening programs facilitate prompt diagnosis. However, the molecular basis for CH remains unclear in most of the cases. This review summarizes current understanding of the genetic etiologies underlying primary CH and associated phenotypes. Classical genetic causes are discussed in the context of their role in normal thyroid physiology. Genes recently reported to play a role in the pathogenesis of CH are discussed, and novel genomic mechanisms in CH are described.
Keywords: congenital hypothyroidism; dyshormonogenesis; genetics; thyroid dysgenesis.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the work reported.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

