Identification of a New FtsZ Inhibitor by Virtual Screening, Mechanistic Insights, and Structure-Activity Relationship Analyses
- PMID: 40100965
- PMCID: PMC11998009
- DOI: 10.1021/acsinfecdis.4c01045
Identification of a New FtsZ Inhibitor by Virtual Screening, Mechanistic Insights, and Structure-Activity Relationship Analyses
Abstract
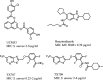
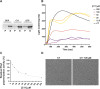
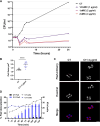
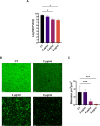
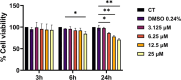
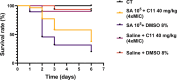
Antimicrobial resistance (AMR) poses a major threat to human health globally. Approximately 5 million deaths were attributed to AMR in 2019, and this figure is predicted to worsen, reaching 10 million deaths by 2050. In the search for new compounds that can tackle AMR, FtsZ inhibitors represent a valuable option. In the present study, a structure-based virtual screening is reported, which led to the identification of derivative C11 endowed with an excellent minimum inhibitory concentration value of 2 μg/mL against Staphylococcus aureus. Biochemical assays clarified that compound C11 targets FtsZ by inhibiting its polymerization process. C11 also showed notable antimicrobial activity against S. aureus cystic fibrosis isolates and methicillin-resistant S. aureus strains. Derivative C11 did not show cytotoxicity, while it had a synergistic effect with methicillin. C11 also showed increased survival in the Galleria mellonella infection model. Lastly, structure-activity relationship and binding mode analyses were reported.
Keywords: FtsZ; antibiotics; antimicrobial resistance; drug discovery; virtual screening.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
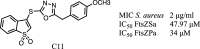

References
-
- Naghavi M.; Mestrovic T.; Gray A.; Gershberg Hayoon A.; Swetschinski L. R.; Robles Aguilar G.; Davis Weaver N.; Ikuta K. S.; Chung E.; Wool E. E.; et al. Global burden associated with 85 pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2024, 24 (8), 868–895. 10.1016/S1473-3099(24)00158-0. - DOI - PMC - PubMed
-
- Beyer P.; Paulin S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect Dis 2020, 6 (6), 1289–1291. 10.1021/acsinfecdis.0c00044. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

