Safety, reactogenicity, and immunogenicity of MTBVAC in infants: a phase 2a randomised, double-blind, dose-defining trial in a TB endemic setting
- PMID: 40101388
- PMCID: PMC12121429
- DOI: 10.1016/j.ebiom.2025.105628
Safety, reactogenicity, and immunogenicity of MTBVAC in infants: a phase 2a randomised, double-blind, dose-defining trial in a TB endemic setting
Abstract
Background: Safer and more effective tuberculosis (TB) vaccines than Bacille Calmette Guérin (BCG) are needed. We evaluated the safety, reactogenicity, and immunogenicity of three dose levels of the live-attenuated Mycobacterium tuberculosis (Mtb) vaccine, MTBVAC, compared to BCG, in South African infants.
Methods: Healthy, HIV-unexposed, BCG-naïve infants were randomised to receive a single intradermal dose of BCG (2.5 × 105 CFU, n = 24) or MTBVAC (2.5 × 104, 2.5 × 105, or 2.5 × 106 CFU, each n = 25). Safety endpoints were solicited systemic, solicited injection site, and unsolicited adverse events (AE), and serious AE (SAE). Immunogenicity was measured using interferon-γ release assay (IGRA) and whole blood intracellular cytokine staining assay. Follow-up was 12 months post-vaccination.
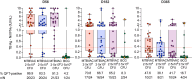
Findings: Ninety-nine infants were enrolled between 18 February 2019 and 08 March 2021. Seventy-eight infants experienced reactogenicity AE (all mild except one grade 2 erythema). Induration, swelling, and erythema were more frequent as MTBVAC dose increased. All reactogenicity events were less frequent in infants receiving MTBVAC 2.5 × 105 CFU compared with BCG. Twelve infants (three BCG and nine MTBVAC recipients) experienced 14 vaccine-unrelated SAE, including one death due to bronchopneumonia (MTBVAC recipient). Eight infants were treated for unconfirmed pulmonary TB (four BCG and four MTBVAC 2.5 × 104 CFU recipients); one BCG recipient was treated for unconfirmed TB meningitis. MTBVAC was immunogenic at all 3 doses, inducing predominantly Th1-cytokine-expressing CD4 T cells, which peaked at Day 56. The 2.5 × 105 and 2.5 × 106 CFU MTBVAC doses induced similar response magnitudes and were more immunogenic than BCG. Day 56 IGRA conversion was observed in 61 (87.4%) infants receiving any MTBVAC dose, but only 28 (42.4%) remained positive by Day 365.
Interpretation: MTBVAC appeared safe, well-tolerated, and immunogenic at doses between 2.5 × 104 and 2.5 × 106 CFU in South African infants. The 2.5 × 105 CFU MTBVAC dose, being less reactogenic and more immunogenic than BCG, was selected for a multi-centre, phase 3 trial.
Funding: This trial was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP).
Keywords: BCG; Infant; Mycobacterium tuberculosis; Tuberculosis; Vaccine.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests ER, JT, EP, JD and IMJ are employed by Biofabri, holders of the EDCTP grant RIA2016V-1637. CM, NA and JGA are employed by University of Zaragosa, the patent holder of “Tuberculosis Vaccine”. NA, JGA and CM declare support to attend MTBVACN3 kickoff meeting in Baiona Spain in 2022 paid by the organisers of the meeting with EDCTP funding. MH declares consultancy for WHO, member of advisory committee TBVI, grant holder for clinical trials through his institution, University of Cape Town. TJS declares grants from Biofabri, EDCTP, NIH CDMRP to his institution, University of Cape Town. MTBVAC202 study group funded by EDCTP grant to institution University of Cape Town. BF received consulting fees as a clinical adviser for the development of MTVAC—payment to BFL Conseils SAS. BF also received support from TBVI to attend meetings related to the study.
Figures


References
-
- WHO . World Health Organisation; Geneva: 2024. Global Tuberculosis report 2024.https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... Available from:
-
- Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367(9517):1173–1180. - PubMed
-
- Mangtani P., Abubakar I., Ariti C., et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

