Vascular network-inspired diffusible scaffolds for engineering functional midbrain organoids
- PMID: 40101722
- PMCID: PMC12327405
- DOI: 10.1016/j.stem.2025.02.010
Vascular network-inspired diffusible scaffolds for engineering functional midbrain organoids
Abstract
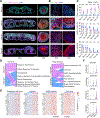
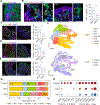
Organoids, 3D organ-like tissue cultures derived from stem cells, show promising potential for developmental biology, drug discovery, and regenerative medicine. However, the function and phenotype of current organoids, especially neural organoids, are still limited by insufficient diffusion of oxygen, nutrients, metabolites, signaling molecules, and drugs. Herein, we present vascular network-inspired diffusible (VID) scaffolds to mimic physiological diffusion physics for generating functional organoids and phenotyping their drug response. Specifically, the VID scaffolds, 3D-printed meshed tubular channel networks, successfully engineer human midbrain organoids almost without necrosis and hypoxia in commonly used well plates. Compared with conventional organoids, these engineered organoids develop more physiologically relevant features and functions, including midbrain-specific identity, oxygen metabolism, neuronal maturation, and network activity. Moreover, these engineered organoids also better recapitulate pharmacological responses, such as neural activity changes to fentanyl exposure, compared with conventional organoids with significant diffusion limits. This platform may provide insights for organoid development and therapeutic innovation.
Keywords: diffusion; drug response; electrophysiology; organoids; perfusion; scaffolds; vascular networks.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The manuscript comes with a patent entitled “Device and methods for engineering perfusable 3D cell cultures” (Application# 63/665,708).
Figures




Update of
-
Vascular network-inspired diffusible scaffolds for engineering functional neural organoids.bioRxiv [Preprint]. 2024 Sep 2:2024.08.31.610649. doi: 10.1101/2024.08.31.610649. bioRxiv. 2024. Update in: Cell Stem Cell. 2025 May 1;32(5):824-837.e5. doi: 10.1016/j.stem.2025.02.010. PMID: 39282292 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

