Mesoscale connectivity of the human hippocampus and fimbria revealed by ex vivo diffusion MRI
- PMID: 40101867
- PMCID: PMC12038723
- DOI: 10.1016/j.neuroimage.2025.121125
Mesoscale connectivity of the human hippocampus and fimbria revealed by ex vivo diffusion MRI
Abstract
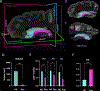
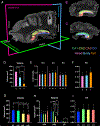
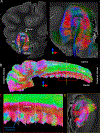
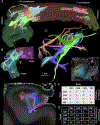
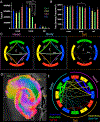
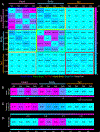
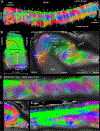
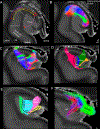
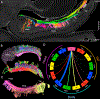
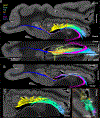
The human hippocampus is essential to cognition and emotional processing. Its function is defined by its connectivity. Although some pathways have been well-established, our knowledge about anterior-posterior connectivity and the distribution of fibers from major fiber bundles remains limited. Mesoscale (250 μm isotropic acquisition, upsampled to 125 μm) resolution MR images of the human temporal lobe afforded a detailed visualization of fiber tracts, including those that related anterior-posterior substructures defined as subregions (head, body, tail) and subfields (cornu ammonis 1-3, dentate gyrus) of the hippocampus. Fifty pathways were dissected between the head and body, highlighting an intricate mesh of connectivity between these two subregions. Along the body subregion, 12 lamellae were identified based on morphology and the presence of interlamellar fibers that appear to connect neighboring lamellae at the edge of the external limb of the granule cell layer (GCL). Translamellar fibers (i.e. longitudinal fibers crossing more than 2 lamellae) were also evident at the edge of the internal limb of the GCL. The dentate gyrus of the body was the main site of connectivity with the fimbria. Unique pathways were dissected within the fimbria that connected the body of the hippocampus with the amygdala and the temporal pole. A topographical segregation within the fimbria was determined by fibers' hippocampal origin, illustrating the importance of mapping the spatial distribution of fibers. Elucidating the detailed structural connectivity of the hippocampus is crucial to develop better diagnostic markers of neurological and psychiatric conditions, as well as to devise novel surgical interventions.
Keywords: Connectome; Diffusion MRI; Fimbria; Hippocampus; Mesoscale; Subfields; Tractography.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no personal financial or institutional interest in the results described in this article.
Figures

References
-
- Abraham H, Vincze A, Jewgenow I, Veszpremi B, Kravjak A, Gomori E, Seress L, 2010. Myelination in the human hippocampal formation from midgestation to adulthood. Int. J. Dev. Neurosci. 28, 401–410. - PubMed
-
- Adelmann G, Deller T, Frotscher M, 1996. Organization of identified fiber tracts in the rat fimbria-fornix: an anterograde tracing and electron microscopic study. Anat. Embryol. 193, 481–493. - PubMed
-
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA, 2018. Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc. Natl. Acad. Sci. USA 115, 4252–4257. - PMC - PubMed
-
- Amaral DG, Witter MP, 1989. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

