[Analgesic effect of "cocktail" analgesia containing high-dose compound betamethasone after revision hip arthroplasty and the use of opioid drugs]
- PMID: 40101906
- PMCID: PMC11919513
- DOI: 10.7507/1002-1892.202412024
[Analgesic effect of "cocktail" analgesia containing high-dose compound betamethasone after revision hip arthroplasty and the use of opioid drugs]
Abstract
Objective: To investigate the analgesic effect of locally injecting a "cocktail" analgesia containing a high-dose compound betamethasone during revision hip arthroplasty, and also to study the usage of opioid drugs.
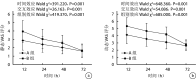
Methods: A retrospective analysis was conducted on the clinical data of 180 patients who underwent revision hip arthroplasty due to aseptic loosening of the hip prosthesis between January 2015 and December 2021. Among them, 95 patients received intraoperative injection of "cocktail" analgesia containing high-dose compound betamethasone (group A), and 85 patients received intraoperative injection of traditional "cocktail" analgesia (group B). There was no significant difference in baseline data such as gender, age, body mass index, presence or absence of diabetes mellitus between the two groups ( P>0.05). The hospital stay, use of opioid drugs within 72 hours, and the incidence of adverse reactions within 72 hours after operation [including nausea and vomiting, insomnia, deep venous thrombosis (DVT), infection, etc.] were recorded and compared between the two groups. The pain relief of patients was evaluated using the static and dynamic visual analogue scale (VAS) scores at 12, 24, 48, and 72 hours after operation. The incidence of complications (including prosthesis re-loosening, hip joint dislocation, hip joint stiffness, limping, chronic pain, etc.) at 2 years after operation was recorded, and the Harris Hip Score (HHS) was used to evaluate the function at 2 years after operation.
Results: In group A, the utilization rate of opioid drugs within 72 hours after operation was significantly lower than that in group B ( P<0.05). However, there was no significant difference between the two groups in terms of hospital stay, as well as the incidence of adverse reactions such as nausea and vomiting, insomnia, DVT, and infection within 72 hours after operation ( P>0.05). The VAS scores of both groups decreased with time, and the differences between different time points were significant ( P<0.05). The static and dynamic VAS scores of group A were significantly lower than those of group B at 12, 24, and 48 hours after operation ( P<0.05), but there was no significant difference in static and dynamic VAS scores between the two groups at 72 hours after operation ( P>0.05). All patients in both groups were followed up 2-8 years, with an average of 5.73 years. At 2 years after operation, no significant difference was found between the two groups in the incidence of complications and HHS score ( P>0.05).
Conclusion: "Cocktail" analgesia containing a high-dose compound betamethasone for early analgesia after revision hip arthroplasty can effectively reduce postoperative pain and the use of opioid drugs, but will not increase the incidence of infection and DVT after operation.
目的: 探讨人工髋关节翻修术中局部应用含大剂量复方倍他米松的“鸡尾酒”注射液镇痛效果及阿片类药物使用情况。.
方法: 回顾分析2015年1月—2021年12月因髋关节假体无菌性松动行人工髋关节翻修术的180例患者临床资料。其中95例患者术中注射含大剂量复方倍他米松的“鸡尾酒”镇痛(A组),85例患者术中注射传统“鸡尾酒”镇痛(B组)。两组患者性别、年龄、身体质量指数、是否合并糖尿病等基线资料比较差异均无统计学意义( P>0.05)。记录并比较两组患者住院时间、术后72 h内阿片类药物使用情况及72 h内不良反应 [包括恶心呕吐、失眠、深静脉血栓形成(deep venous thrombosis,DVT)、感染等]发生情况;术后12、24、48、72 h采用静态和动态疼痛视觉模拟评分(VAS)评估患者疼痛缓解情况;记录术后2年并发症 [包括假体再次松动、髋关节脱位、髋关节僵硬、跛行、慢性疼痛等]发生情况,采用Harris髋关节评分(HHS)评价术后2年功能。.
结果: A组术后72 h内阿片类药物使用率低于B组,差异有统计学意义( P<0.05);两组住院时间及术后72 h内恶心呕吐、失眠、DVT、感染等不良反应发生率比较差异均无统计学意义( P>0.05)。术后两组VAS评分均随时间延长逐渐降低,各时间点差异均有统计学意义( P<0.05);术后12、24、48 h A组静态和动态VAS评分均低于B组,差异有统计学意义( P<0.05);术后72 h两组静态和动态VAS评分比较差异均无统计学意义( P>0.05)。两组患者均获随访,随访时间2~8年,平均5.73年。术后2年两组并发症发生情况及HHS评分比较差异均无统计学意义( P>0.05)。.
结论: 人工髋关节翻修术中使用含大剂量复方倍他米松的“鸡尾酒”,可有效缓解术后早期疼痛及减少阿片类药物的使用,且不会增加术后感染、DVT等并发症发生率。.
Keywords: Revision hip arthroplasty; analgesia; compound betamethasone; “cocktail”.
Conflict of interest statement
利益冲突 在课题研究和文章撰写过程中不存在利益冲突;经费支持没有影响文章观点和对研究数据客观结果的统计分析及其报道
Figures
References
-
- 努尔艾力江·玉山, 张晓岗, 吾湖孜·吾拉木, 等 含大剂量复方倍他米松鸡尾酒注射液在一期翻修治疗假体周围感染中的应用及安全性研究. 骨科. 2024;15(4):289–295.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical