Progesterone receptor-dependent downregulation of MHC class I promotes tumor immune evasion and growth in breast cancer
- PMID: 40102028
- PMCID: PMC11927445
- DOI: 10.1136/jitc-2024-010179
Progesterone receptor-dependent downregulation of MHC class I promotes tumor immune evasion and growth in breast cancer
Abstract
Background: Breast cancer (BC) continues to be a major health concern with 250,000 new cases diagnosed annually in the USA, 75% of which are hormone receptor positive (HR+), expressing estrogen receptor alpha (ER) and/or the progesterone receptor (PR). Although ER-targeted therapies are available, 30% of patients will develop resistance, underscoring the need for new non-ER/estrogen-based treatments. Notably, HR+BCs exhibit poor lymphocyte infiltration and contain an immunosuppressive microenvironment, which contributes to the limited efficacy of immunotherapies in HR+BC. In this study, we demonstrate that PR/progesterone signaling reduces major histocompatibility complex (MHC) Class I expression, facilitating immune evasion and escape from immune-based clearance of PR+tumors.
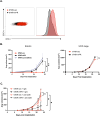
Methods: To determine the effect of PR/progesterone on MHC Class I expression, we treated human and mouse mammary tumor cell lines with progesterone and/or interferon (IFN) and measured expression of genes involved in antigen processing and presentation (APP), as well as surface MHC Class I expression. We used the OT-I/SIINFEKL model antigen system to measure the impact of progesterone on immune cell-mediated killing of modified tumor cells. We also analyzed two large BC clinical cohorts to determine how PR expression correlates with APP gene expression and MHC Class I expression in ER-positive tumors.
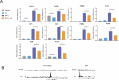
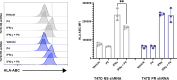
Results: In vitro, we show that PR/progesterone signaling reduces APP gene expression and MHC class I expression in human and breast mammary tumor cell lines. PR-mediated attenuation of APP/MHC Class I expression is more pronounced in the presence of IFN. In immune cell killing assays, PR-expressing mammary tumor cells treated with progesterone are protected from immune-mediated cytotoxicity. We demonstrate that PR expression in vivo prevents immune-mediated rejection of xenoantigen-modified mammary tumor cell lines through mechanisms involving MHC Class I expression and CD8 T cells. Data analysis of two large BC cohorts reveals lower APP gene expression and MHC Class I expression in ER/PR-positive tumors compared with ER-positive/PR-negative tumors. These findings show that HR+BCs, specifically PR+tumors, downregulate APP/MHC class I machinery through PR/progesterone signaling. Use of pharmacological PR/progesterone inhibitors may reverse these effects in patients with BC, thereby improving immunosurveillance and response to immunotherapies.
Keywords: Breast Cancer; Immunosuppression; Major histocompatibility complex - MHC.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: CRH received funds from Context Therapeutics (former developer of onapristone). JMB receives research support from Genentech/Roche and Incyte Corporation, has received advisory board payments from AstraZeneca, Eli Lilly, and Mallinckrodt, and is an inventor on patents regarding immunotherapy targets and biomarkers in cancer. The remaining authors have nothing to disclose.
Figures


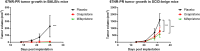

References
-
- Atlanta: American Cancer Society; 2024. Cancer facts & figures 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
